11-Dehydrothromboxane B2
 | |
| Names | |
|---|---|
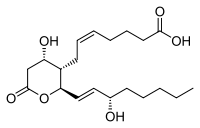
| IUPAC name
(Z)-7-[(2R,3S,4S)-4-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-6-oxo-tetrahydropyran-3-yl]hept-5-enoic acid | |
| Other names
11-Dehydro-thromboxane B2 | |
| Identifiers | |
| 67910-12-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28667 |
| ChemSpider | 4444414 |
| 4484 | |
| KEGG | C05964 |
| PubChem | 5280891 |
| |
| |
| Properties | |
| C20H32O6 | |
| Molar mass | 368.46 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
11-Dehydrothromboxane B2 (or 11-dehydro-TXB2) is produced from the breakdown of thromboxane A2. It is released by activated platelets and urine levels of 11-dehydro-TXB2 can be used to monitor the response to aspirin therapy when used to prevent heart disease[1] and in diseases where platelet activation is prominent.[2]
References
- ↑ Lordkipanidzé M, Pharand C, Schampaert E, Turgeon J, Palisaitis DA, Diodati JG (2007). "A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease". Eur Heart J. 28 (14): 1702–1708. doi:10.1093/eurheartj/ehm226. PMID 17569678.
- ↑ Catella F, Healy D, Lawson JA, FitzGerald GA (1986). "11-Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation". PNAS. 83 (16): 5861–5865. doi:10.1073/pnas.83.16.5861. PMC 386396
 . PMID 3461463.
. PMID 3461463.
This article is issued from Wikipedia - version of the 5/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.