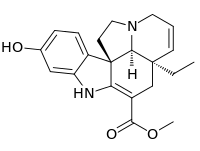
16-Hydroxytabersonine
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (5α,12β,19α)-16-hydroxy-2,3,6,7-tetradehydroaspidospermidine-3-carboxylate | |
| Other names
11-Hydroxytabersonine | |
| Identifiers | |
| 22149-28-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 391562 |
| PubChem | 443326 |
| |
| |
| Properties | |
| C21H24N2O3 | |
| Molar mass | 352.43 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
16-Hydroxytabersonine is a terpene indole alkaloid produced by the plant Catharanthus roseus. The metabolite is an intermediate in the formation of vindoline, a precursor needed for formation of the pharmaceutically valuable vinblastine and vincristine. 16-hydroxytabersonine is formed from the hydroxylation of tabersonine by tabersonine 16-hydroxylase (T16H). Tabersonine 16-O-methyltransferase (16OMT) methylates the hydroxylated 16 position to form 16-methoxytabersonine.[1]
References
- ↑ Levac, Murata, Kim and De Luca (2008) Application of carborundum abrasion for investigating the leaf epidermis: molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. The Plant Journal. 53(2). 225-236
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.