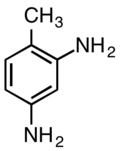
2,4-Diaminotoluene
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methylbenzene-1,3-diamine | |
| Other names
2,4-Toluenediamine | |
| Identifiers | |
| 95-80-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 6991 |
| ECHA InfoCard | 100.002.231 |
| PubChem | 7261 |
| |
| Properties | |
| C7H10N2 | |
| Molar mass | 122.17 g·mol−1 |
| Appearance | White solid |
| Density | 1.521 g/cm3 |
| Melting point | 97 to 99 °C (207 to 210 °F; 370 to 372 K) |
| Boiling point | 283 to 285 °C (541 to 545 °F; 556 to 558 K) |
| Hazards | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
Ca[1] |
| IDLH (Immediate danger) |
Ca [N.D.][1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4-Diaminotoluene is an organic compound with the formula C6H3(NH2)2CH3. It is one isomer of six with this formula. It is a white solid although commercial samples are often yellow-tan. It is prepared by hydrogenation of 2,4-dinitrotoluene using a nickel catalyst. Commercial samples often contain up to 20% of the 2,6-isomer.[2]
It is mainly used as a precursor to toluene diisocyanate, a precursor to polyurethane.
Its reaction with benzenediazonium chloride gives the cationic azo dye Basic Orange 1. Condensation of 2,4-diaminotoluene with acetaldehyde gives the acridine dye called Basic Yellow 9.[3]

Synthesis of C.I. Basic Yellow 9, an acridine dye.
References
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0620". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
- ↑ Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a27_179
This article is issued from Wikipedia - version of the 9/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.