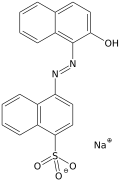
Acid red 88
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium 4-(2-hydroxy-1-naphthalenylazo)-naphthalenesulfonate | |
| Other names
Fast Red A 2-Naphthol Red | |
| Identifiers | |
| 1658-56-6 (E)-diazen | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23621588 |
| ECHA InfoCard | 100.015.238 |
| EC Number | 216-760-3 |
| MeSH | Fast+red+S |
| PubChem | 23722700 23669381 (Z)-diazen 23670762 (E)-diazen |
| RTECS number | QK2420000 |
| |
| |
| Properties | |
| C20H13N2NaO4S | |
| Molar mass | 400.38 g·mol−1 |
| Appearance | Vivid, dark red, opaque, vitreous solid |
| Melting point | 280 °C (536 °F; 553 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Acid red 88 (also named Solid Red A, 2-Naphthol Red, Toyo roccelline, Fast Red A and so on) is a red azo dye. Due to its intense colour it looks almost black when solid. Instead of crystallising, it vitrifies when cooled or salted out of the solution. It has a pungent smell similar to that of benzoic acid.
Preparation
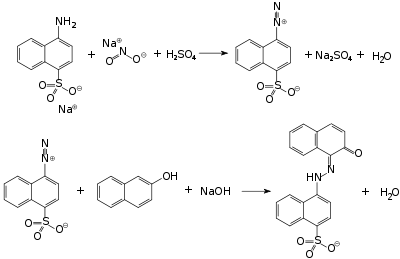
It can be obtained by azo coupling of naphthionic acid and 2-naphthol.
Uses
This compound is used in the textile industry as a dye.[1] It can also be used for research in photocatalysis (as degradation object).[2]
References
External links
- echo Chemical Database: 1-Naphthalenesulfonic acid, 4-((2-hydroxy-1-naphthalenyl)azo)-, monosodium salt (EnvironmentalChemistry.com)- This page contains information on the chemical 1-Naphthalenesulfonic acid, 4-((2-hydroxy-1-naphthalenyl)azo)-, monosodium salt including: 72 synonyms/identifiers.
This article is issued from Wikipedia - version of the 6/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.