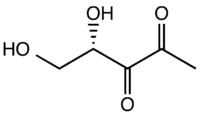
4,5-Dihydroxy-2,3-pentanedione
 | |
| Names | |
|---|---|
| Other names
1-Deoxypento-2,4-diulose; 1-Deoxypentosone; Dihydroxy-2,3-pentanedione, DPD | |
| Identifiers | |
| 142937-55-1 | |
| Properties | |
| C5H8O4 | |
| Molar mass | 132.12 g·mol−1 |
| Appearance | Colorless |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4,5-Dihydroxy-2,3-pentanedione (DPD) is an organic compound that occurs naturally but exists as several related structures. The idealized formula for this species is CH3C(O)C(O)CH(OH)CH2OH, but it is known to exist as several other forms resulting from cyclization. It is not stable at room temperature as a pure material, which has further complicated its analysis. The (S)-stereoisomer occurs naturally. It is typically hydrated, i.e., one keto group has added water to give the geminal diol.
DPD is produced by degradation of S-adenosylhomocysteine by the action of the enzyme S-ribosylhomocysteinase.[1] The compound probably does not exist as depicted above, but as an equilibrium mixture of three hydrates.
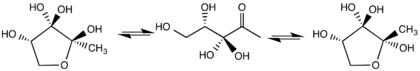
DPD is a precursor to borate diester, which is known as autoinducer-2 (AI-2). AI-2 is a signaling molecules found in quorum sensing. It is produced and recognized by many Gram-negative and Gram-positive bacteria.[3][4] AI-2 is synthesized by the reaction of 1-deoxy-3-dehydro-D-ribulose with boric acid [5] and is recognized by the two-component sensor kinase LuxPQ in Vibrionaceae.
References
- ↑ Jinge Zhu, Eric Dizin, Xubo Hu, Anne-Sophie Wavreille, Junguk Park, Dehua Pei "S-Ribosylhomocysteinase (LuxS) Is a Mononuclear Iron Protein" Biochemistry, 2003, volume 42, pp 4717–4726. doi:10.1021/bi034289j
- ↑ Roberta J. Worthington and Christian Melander "Deconvoluting Interspecies Bacterial Communication" Angew. Chem. Int. Ed. 2012, volume 51, 6314 – 6315. doi:10.1002/anie.201202440
- ↑ Miller, Stephen T.; Xavier, Karina B.; Campagna, Shawn R.; Taga, Michiko E.; Semmelhack, Martin F.; Bassler, Bonnie L.; Hughson, Frederick M. (2004). "Salmonella typhimurium Recognizes a Chemically Distinct Form of the Bacterial Quorum-Sensing Signal AI-2". Molecular Cell. 15 (5): 677–687. doi:10.1016/j.molcel.2004.07.020. PMID 15350213.
- ↑ Miller, M. B.; Bassler, B. L. (2001). "Quorum sensing in bacteria". Annual Review of Microbiology. 55: 165–199. doi:10.1146/annurev.micro.55.1.165. PMID 11544353.
- ↑ http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/misc/AI2.html