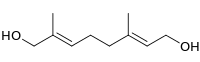
8-Hydroxygeraniol
 | |
| Names | |
|---|---|
| IUPAC name
(2E,6E)-2,6-Dimethyl-2,6-octadiene-1,8-diol | |
| Identifiers | |
| 26488-97-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4515751 |
| ECHA InfoCard | 100.209.637 |
| PubChem | 5363397 |
| |
| |
| Properties | |
| C10H18O2 | |
| Molar mass | 170.25 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
8-Hydroxygeraniol (also called 10-hydroxygeraniol)[1] is a monoterpene synthesized from geraniol by the enzyme geraniol 8-hydroxylase. 8-Hydroxygeraniol is a substrate for 8-hydroxygeraniol dehydrogenase (G80) which synthesizes 8-oxogeranial.[2] 8-Hydroxygeraniol is step in the synthesis of the secologanin, a key monoterpene needed for formation of terpene indole alkaloids.
References
- ↑ Dewick (2009) Medicinal Natural Products: A Biosynthetic Approach.
- ↑ Miettinen, Karel; Dong, Lemeng; Navrot, Nicolas; Schneider, Thomas; Burlat, Vincent; Pollier, Jacob; Woittiez, Lotte; Van Der Krol, Sander; Lugan, Raphaël; Ilc, Tina; Verpoorte, Robert; Oksman-Caldentey, Kirsi-Marja; Martinoia, Enrico; Bouwmeester, Harro; Goossens, Alain; Memelink, Johan; Werck-Reichhart, Danièle (2014). "The seco-iridoid pathway from Catharanthus roseus". Nature Communications. 5. Bibcode:2014NatCo...5.....M. doi:10.1038/ncomms4606.
This article is issued from Wikipedia - version of the 6/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.