Acetic formic anhydride
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic formic anhydride | |
| Other names | |
| Identifiers | |
| 2258-42-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 67812 |
| PubChem | 75269 |
| |
| |
| Properties | |
| C3H4O3 | |
| Molar mass | 88.06 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
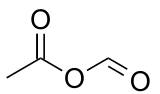
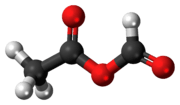
Acetic formic anhydride, or ethanoic methanoic anhydride is an organic compound with the chemical formula C
3H
4O
3 and a structural formula of H
3C-(C=O)-O-(C=O)H. It can be viewed as the mixed anhydride of acetic acid and formic acid.
Preparation
Acetic formic anhydride can be produced by reacting sodium formate with acetyl chloride in anhydrous diethyl ether between 23–27 °C.[2]
Applications
Acetic formic anhydride is a formylation agent for amines, amino acids, and alcohols. It is also a starting material for other compounds such as formyl fluoride.[2]
An example of Acetic formic anhydride being used was in the synthesis of Quazodine (U.S. Patent 3,248,292).
See also
References
- ↑ "Formyl acetate - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- 1 2 Krimen, Lewis I. (1970). "Acetic Formic Anhydride". Organic Syntheses. 50: 1. doi:10.15227/orgsyn.050.0001.
This article is issued from Wikipedia - version of the 9/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.