Adiabatic flame temperature
In the study of combustion, there are two types of adiabatic flame temperature depending on how the process is completed, constant volume and constant pressure, describing the temperature the combustion products theoretically reach if no energy is lost to the outside environment.
The constant volume adiabatic flame temperature is the temperature that results from a complete combustion process that occurs without any work, heat transfer or changes in kinetic or potential energy. The constant pressure adiabatic flame temperature is the temperature that results from a complete combustion process that occurs without any heat transfer or changes in kinetic or potential energy. Its temperature is lower than the constant volume process because some of the energy is utilized to change the volume of the system (i.e., generate work).
Common flames
In daily life, the vast majority of flames one encounters are those of organic compounds including wood, wax, fat, common plastics, propane, and gasoline. The constant-pressure adiabatic flame temperature of such substances in air is in a relatively narrow range around 1950 °C. This is because, in terms of stoichiometry, the combustion of an organic compound with n carbons involves breaking roughly 2n C–H bonds, n C–C bonds, and 1.5n O2 bonds to form roughly n CO2 molecules and n H2O molecules.
Because most combustion processes that happen naturally occur in the open air, there is nothing that confines the gas to a particular volume like the cylinder in an engine. As a result, these substances will burn at a constant pressure allowing the gas to expand during the process.
Common flame temperatures
Assuming initial atmospheric conditions (1 bar and 20 °C), the following table list the adiabatic flame temperature for various gases under constant pressure conditions. The temperatures mentioned here are for a stoichiometric fuel-oxidizer mixture (i.e. equivalence ratio φ = 1).
Note these are theoretical, not actual, flame temperatures produced by a flame that loses no heat. The closest will be the hottest part of a flame, where the combustion reaction is most efficient. This also assumes complete combustion (e.g. perfectly balanced, non-smokey, usually bluish flame)
| Fuel | Oxidizer | (°C) | (°F) |
|---|---|---|---|
| Acetylene (C2H2) | Air | 2500 | 4532 |
| Acetylene (C2H2) | Oxygen | 3480 | 6296 |
| Butane (C4H10) | Air | 1970 | 3578 |
| Cyanogen (C2N2) | Oxygen | 4525 | 8177 |
| Dicyanoacetylene (C4N2) | Oxygen | 4990 | 9010 |
| Ethane (C2H6) | Air | 1955 | 3551 |
| Ethanol (C 2H 5OH) | Air | 2082 | 3779 [1] |
| Gasoline | Air | 2138 | 3880 [1] |
| Hydrogen (H2) | Air | 2254 | 4089 [1] |
| Hydrogen (H2) | Oxygen | 3200 | 5792 [2] |
| Methane (CH4) | Air | 1963 | 3565 [3] |
| Methanol (CH4O) | Air | 1949 | 3540 [3] |
| Natural gas | Air | 1960 | 3562 [4] |
| Pentane (C5H12 | Air | 1977 | 3591 [3] |
| Propane (C3H8) | Air | 1980 | 3596 [5] |
| Propane (C3H8) | Oxygen | 2526 | 4579 |
| MAPP gas Methylacetylene (C3H4) | Air | 2010 | 3650 |
| MAPP gas Methylacetylene (C3H4) | Oxygen | 2927 | 5301 |
| Toluene (C7H8) | Air | 2071 | 3760 [3] |
| Wood | Air | 1980 | 3596 |
| Kerosene | Air | 2093 [6] | 3801 |
| Light fuel oil | Air | 2104 [6] | 3820 |
| Medium fuel oil | Air | 2101 [6] | 3815 |
| Heavy fuel oil | Air | 2102 [6] | 3817 |
| Bituminous Coal | Air | 2172 [6] | 3943 |
| Anthracite | Air | 2180 [6] | 3957 |
| Anthracite | Oxygen | ≈2900 [see 1] | ≈5255 |
| Aluminum | Oxygen | 3732 | 6750 [3] |
| Lithium | Oxygen | 2438 | 4420 [3] |
| Phosphorus (white) | Oxygen | 2969 | 5376 [3] |
| Zirconium | Oxygen | 4005 | 7241 [3] |
- ↑ The temperature equal to ≈3200 K corresponds to 50 % of chemical dissociation for CO2 at pressure 1 atm. The latter one stays invariant for adiabatic flame and the carbon dioxide constitutes 97 % of total gas output in the case of anthracite burning in oxygen. Higher temperatures will occur for reaction output while it going under higher pressure (up to 3800 K and above, see e.g. Jongsup Hong et al, p.8).
Thermodynamics
.jpg)
From the first law of thermodynamics for a closed reacting system we have,
where, and are the heat and work transferred from the system to the surroundings during the process respectively, and and are the internal energy of the reactants and products respectively. In the constant volume adiabatic flame temperature case, the volume of the system is held constant hence there is no work occurring,
and there is no heat transfer because the process is defined to be adiabatic: . As a result, the internal energy of the products is equal to the internal energy of the reactants: . Because this is a closed system, the mass of the products and reactants is constant and the first law can be written on a mass basis,
- .
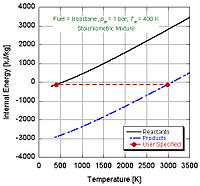
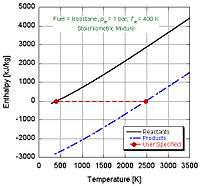
In the constant pressure adiabatic flame temperature case, the pressure of the system is held constant which results in the following equation for the work,
Again there is no heat transfer occurring because the process is defined to be adiabatic: . From the first law, we find that,
Recalling the definition of enthalpy we recover: . Because this is a closed system, the mass of the products and reactants is constant and the first law can be written on a mass basis,
- .
We see that the adiabatic flame temperature of the constant pressure process is lower than that of the constant volume process. This is because some of the energy released during combustion goes into changing the volume of the control system. One analogy that is commonly made between the two processes is through combustion in an internal combustion engine. For the constant volume adiabatic process, combustion is thought to occur instantaneously when the piston reaches the top of its apex (Otto cycle or constant volume cycle). For the constant pressure adiabatic process, while combustion is occurring the piston is moving in order to keep the pressure constant (Diesel cycle or constant pressure cycle).
.jpg)
.jpg)
If we make the assumption that combustion goes to completion (i.e. and ), we can calculate the adiabatic flame temperature by hand either at stoichiometric conditions or lean of stoichiometry (excess air). This is because there are enough variables and molar equations to balance the left and right hand sides,
Rich of stoichiometry there are not enough variables because combustion cannot go to completion with at least and needed for the molar balance (these are the most common incomplete products of combustion),
However, if we include the Water gas shift reaction,
and use the equilibrium constant for this reaction, we will have enough variables to complete the calculation.
Different fuels with different levels of energy and molar constituents will have different adiabatic flame temperatures.
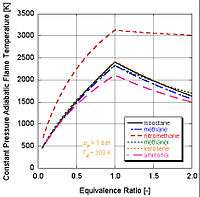
.jpg)
We can see by the following figure why nitromethane (CH3NO2) is often used as a power boost for cars. Since each mole of nitromethane contains two moles of oxygen, it can burn much hotter because it provides its own oxidant along with fuel. This in turn allows it to build up more pressure during a constant volume process. The higher the pressure, the more force upon the piston creating more work and more power in the engine. It is interesting to note that it stays relatively hot rich of stoichiometry because it contains its own oxidant. However, continual running of an engine on nitromethane will eventually melt the piston and/or cylinder because of this higher temperature.
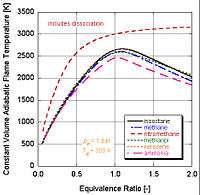
In real world applications, complete combustion does not typically occur. Chemistry dictates that dissociation and kinetics will change the relative constituents of the products. There are a number of programs available that can calculate the adiabatic flame temperature taking into account dissociation through equilibrium constants (Stanjan, NASA CEA, AFTP). The following figure illustrates that the effects of dissociation tend to lower the adiabatic flame temperature. This result can be explained through Le Chatelier's principle.
See also
References
- 1 2 3 Flame Temperature Analysis and NOx Emissions for Different Fuels
- ↑ Flame temperatures
- 1 2 3 4 5 6 7 8 CRC Handbook of Chemistry and Physics, 96th Edition, p. 15-51
- ↑ North American Combustion Handbook, Volume 1, 3rd edition, North American Mfg Co., 1986.
- ↑
- 1 2 3 4 5 6 Power Point Presentation: Flame Temperature, Hsin Chu, Department of Environmental Engineering, National Cheng Kung University, Taiwan
External links
General information
- Babrauskas, Vytenis (2006-02-25). "Temperatures in flames and fires". Fire Science and Technology Inc. Archived from the original on 12 January 2008. Retrieved 2008-01-27.
- Computation of adiabatic flame temperature
- Adiabatic flame temperature
Tables
- "Adiabatic Flame Temperature". The Engineering Toolbox. Archived from the original on 28 January 2008. Retrieved 2008-01-27. adiabatic flame temperature of hydrogen, methane, propane and octane with oxygen or air as oxidizers
- "Flame Temperatures for some Common Gases". The Engineering Toolbox. Archived from the original on 7 January 2008. Retrieved 2008-01-27.
- Temperature of a blue flame and common materials
Calculators
- Online adiabatic flame temperature calculator using Cantera
- Adiabatic flame temperature program
- Gaseq, program for performing chemical equilibrium calculations.
- Flame Temperature Calculator - Constant pressure bipropellant adiabatic combustion
- Adiabatic Flame Temperature calculator