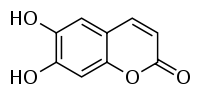
Aesculetin
 | |
| Names | |
|---|---|
| IUPAC name
6,7-dihydroxy-chromen-2-one | |
| Other names
esculetin cichorigenin 6,7-dihydroxycoumarin | |
| Identifiers | |
| 305-01-1 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:490095 |
| ChEMBL | ChEMBL244743 |
| ChemSpider | 4444764 |
| ECHA InfoCard | 100.005.602 |
| 5180 | |
| KEGG | C09263 |
| PubChem | 5281416 |
| |
| |
| Properties | |
| C9H6O4 | |
| Molar mass | 178.14 g mol−1 |
| Appearance | white or light yellow powder |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Aesculetin (also known as esculetin, 6,7-dihydroxycoumarin and cichorigenin) is a derivative of coumarin. It is a natural lactone that derives from the intramolecular cyclization of a cinnamic acid derivative.
It is present in chicory and in many toxic and medicinal plants, in form of glycosides and caffeic acid conjugates.[2]
Esculetin-containing preparations used systemically can have an anticoagulant effect and might interact with anticoagulent drugs such as warfarin.
This compound is used in some sunscreens, but there is evidence that it acts as a photosensitizer for DNA damage.[3] The sodium salt of its methyl-derivative is used in dermatology for the treatment of varicose veins.[4]
It is a blue fluorescence compound found in plants.[5] Aesculin, the glucoside of aesculetin, will fluoresce under long wave ultraviolet light (360 nm). The hydrolysis of aesculin results in loss of this fluorescence. Aesculetin has the ability to quench the inner fluorescence of bovine serum albumin.[6]
Aesculetin can be transformed into scopoletin (7-hydroxy-6-methoxycoumarin) and isoscopoletin (6-hydroxy-7-methoxycoumarin) through incubation with rat liver catechol-O-methyltransferase.[7]
See also
References
- ↑ "Aesculetin". Sigma-Aldrich.
- ↑ Dey, P. M.; Harborne, J. B., eds. (1997). Plant Biochemistry. Academic Press. ISBN 9780122146749.
- ↑ Hausen, B. M.; Schmieder, M. (September 1986). "The sensitizing capacity of coumarins (I)". Contact Dermatitis. 15 (3): 157–163. doi:10.1111/j.1600-0536.1986.tb01317.x. PMID 3780217.
- ↑ ""Permethol" Data Sheet" (PDF).
- ↑ Lang, M.; Stober, F.; Lichtenthaler, H. K. (1991). "Fluorescence emission spectra of plant leaves and plant constituents". Radiation and Environmental Biophysics. 30 (4): 333–347. doi:10.1007/BF01210517.
- ↑ Liu, X.-F.; Xia, Y.-M.; Fang, Y.; Zou, L.; Liu, L.-L. (2004). "Interaction between natural pharmaceutical homologues of coumarin and bovine serum albumin". Huaxue xuebao. 62 (16): 1484–1490. INIST:16312595
- ↑ Müller-Enoch, D.; Seidl, E.; Thomas, H. (1976). "6.7-Dihydroxycoumarin (Aesculetin) as a substrate for catechol-o-methyltransferase". Z. Naturforsch. C (in German). 31 (5-6): 280–284. PMID 134569.