Amlexanox
 | |
| Clinical data | |
|---|---|
| Trade names | Aphthasol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601017 |
| Routes of administration | Topical |
| ATC code | A01AD07 (WHO) R03DX01 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | 3.5 hours |
| Excretion | Renal (17%) |
| Identifiers | |
| |
| CAS Number |
68302-57-8 |
| PubChem (CID) | 2161 |
| IUPHAR/BPS | 7113 |
| DrugBank |
DB01025 |
| ChemSpider |
2076 |
| UNII |
BRL1C2459K |
| KEGG |
D01828 |
| ChEBI |
CHEBI:31205 |
| ChEMBL |
CHEMBL1096 |
| Chemical and physical data | |
| Formula | C16H14N2O4 |
| Molar mass | 298.293 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Amlexanox (trade name Aphthasol) is an anti-inflammatory antiallergic immunomodulator used to treat recurrent aphthous ulcers (canker sores), and (in Japan) several inflammatory conditions. This drug has been discontinued in the U.S.[1]
Medical uses
Amlexanox is the active ingredient in a common topical treatment for recurrent aphthous ulcers of the mouth (canker sores),[2] reducing both healing time[3] and pain.[4] Amlexanox 5% paste is well-tolerated,[5] and is typically applied four times per day directly on the ulcers.[3] A 2011 review found it to be the most effective treatment of the eight treatments investigated for recurrent canker sores.[6] It is also used to treat ulcers associated with Behçet disease.[7]
In Japan, it is used to treat bronchial asthma, allergic rhinitis and conjunctivitis.[8]
Although it is one of the only effective treatments known for apthous stomatitis, the company that claims to have the rights to the drug, Uluru Inc., is either unwilling or incapable of manufacturing it. The company has not responded to patients who have pleaded for help in obtaining the medication for relief from this often debilitating condition.
Contraindications
The drug is contraindicated in those with known allergies to it.[3]
Adverse effects
Amlexanox may cause a slightly painful stinging or burning sensation, nausea or diarrhea.[3]
Mechanism of action
The drug is an anti-inflammatory,[8] antiallergic[9] immunomodulator.[10]
Its mechanism of action is not well-determined, but it might inhibit inflammation by inhibiting the release of histamine and leukotrienes.[8] It has been shown to selectively inhibt TBK1 and IKK-ε, producing reversible weight loss and improved insulin sensitivity, reduced inflammation and attenuated hepatic steatosis without affecting food intake in obese mice.[11]
Chemistry
The chemical itself is an odorless, white to yellowish-white powder.[8]
The 5% preparation for patient use is an adherent beige paste,[3][8] and it is also available in some countries as a tablet that adheres to the ulcer in the mouth.[4]
Pharmacokinetics
Amlexanox applied to an aphthous ulcer is largely absorbed through the gastrointestinal tract; an insignificant amount enters the bloodstream through the ulcer itself. After a single 100 mg dose, mean maximum serum concentration occurs 2.4 +/- 0.9 hours after application, with a half-life of elimination (through urine) of 3.5 +/- 1.1 hours. With multiple daily applications (four doses per day), steady state serum levels occur after one week, with no accumulation occurring after four weeks.[8]
History
The patent for its use as a treatment for aphthous ulcers was issued in November 1994 to inventors Kakubhai R. Vora, Atul Khandwala and Charles G. Smith, and assigned to Chemex Pharmaceuticals, Inc.[12]
Society and culture
Legal status
A prescription is required to obtain the medication.[13]
Economics
A 2011 review found a one-week supply of amlexanox 5% paste to cost $30.[6]
Research
A review found that, as of July 2011, robust studies investigating its effectiveness alongside other canker sore treatments were still needed.[14]
Because it is an inhibitor of the protein kinases TBK1 and IKK-ε,[11] which are implicated in the etiology of type II diabetes and obesity,[15] amlexanox may be a candidate for human clinical trials testing in relation to these diseases.[11]
Synthesis
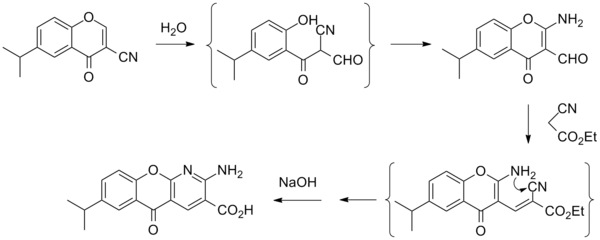
References
- ↑ "Amlexanox (Aphthasol®)". Retrieved 20 November 2013.
- ↑ Gonsalves WC, Chi AC, Neville BW (February 2007). "Common oral lesions: Part I. Superficial mucosal lesions". Am Fam Physician. 75 (4): 501–7. PMID 17323710.
- 1 2 3 4 5 "Amlexanox". MedlinePlus. U.S. National Library of Medicine. February 2009. Retrieved 12 February 2013.
- 1 2 Plewa MC (March 2012). "Pediatric Aphthous Ulcers Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- ↑ "Amlexanox". PubChem. U.S. National Library of Medicine. Retrieved 12 February 2013.
- 1 2 Bailey J, McCarthy C, Smith RF (October 2011). "Clinical inquiry. What is the most effective way to treat recurrent canker sores?". J Fam Pract. 60 (10): 621–32. PMID 21977491.
- ↑ Yousefi M, Ferringer T, Lee S, Bang D (July 2012). "Dermatologic Aspects of Behcet Disease Treatment & Management". Medscape Reference. Medscape. Retrieved 14 February 2013.
- 1 2 3 4 5 6 Bell J (2005). "Amlexanox for the treatment of recurrent aphthous ulcers". Clin Drug Investig. 25 (9): 555–66. doi:10.2165/00044011-200525090-00001. PMID 17532700.
- ↑ "Amlexanox". MeSH. U.S. National Library of Medicine. 2009. Retrieved 12 February 2013.
- ↑ Elad S, Epstein JB, von Bültzingslöwen I, Drucker S, Tzach R, Yarom N (March 2011). "Topical immunomodulators for management of oral mucosal conditions, a systematic review; Part II: miscellaneous agents". Expert Opin Emerg Drugs. 16 (1): 183–202. doi:10.1517/14728214.2011.528390. PMID 21244328.
- 1 2 3 Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR (February 2013). "An inhibitor of the protein kinases TBK1 and IKK-ɛ improves obesity-related metabolic dysfunctions in mice". Nat. Med. 19 (3): 313–21. doi:10.1038/nm.3082. PMID 23396211.
- ↑ US patent 5362737, Kakubhai R. Vora, Atul Khandwala, Charles G. Smith, "Methods of treating aphthous ulcers and other mucocutaneous disorders with amlexanox", assigned to Chemex Pharmaceuticals, Inc.
- ↑ Burgess JA, van der Ven PF, Martin M, Sherman J, Haley J (2008). "Review of over-the-counter treatments for aphthous ulceration and results from use of a dissolving oral patch containing glycyrrhiza complex herbal extract". J Contemp Dent Pract. 9 (3): 88–98. PMID 18335124.
- ↑ Kuteyi T, Okwundu CI (2012). Kuteyi, Teslim, ed. "Topical treatments for HIV-related oral ulcers". Cochrane Database Syst Rev. 1: CD007975. doi:10.1002/14651858.CD007975.pub2. PMID 22258979.
- ↑ Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, Delproposto JB, Blackwell TS, Yull FE, Saltiel AR (September 2009). "The protein kinase IKKepsilon regulates energy balance in obese mice". Cell. 138 (5): 961–75. doi:10.1016/j.cell.2009.06.046. PMC 2756060
 . PMID 19737522.
. PMID 19737522. - ↑ Nohara, A.; Ishiguro, T.; Ukawa, K.; Sugihara, H.; Maki, Y.; Sanno, Y. (1985). "Studies on Antianaphylactic Agents. 7. Synthesis of Antiallergic 5-Oxo-5H-\1]benzopyrano\2,3-b]pyridines". Journal of Medicinal Chemistry. 28 (5): 559–568. doi:10.1021/jm50001a005. PMID 3989816.