Benzyl benzoate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzyl benzoate | |
| Other names
Ascabin Ascabiol Ascarbin Benzylate Scabanca Tenutex Vanzoate Venzoate Benzoic acid phenylmethyl ester Benzy alcohol benzoic ester | |
| Identifiers | |
| 120-51-4 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | BnBzO |
| ChEBI | CHEBI:41237 |
| ChEMBL | ChEMBL1239 |
| ChemSpider | 13856959 |
| DrugBank | DB02775 |
| ECHA InfoCard | 100.004.003 |
| KEGG | D01138 |
| PubChem | 2345 |
| UNII | N863NB338G |
| |
| |
| Properties | |
| C14H12O2 | |
| Molar mass | 212.25 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | faint aromatic |
| Density | 1.118 g/cm3 |
| Melting point | 18 °C (64 °F; 291 K) |
| Boiling point | 323 °C (613 °F; 596 K) |
| insoluble | |
| Solubility | miscible in alcohol, chloroform, ether, oils soluble in acetone, benzene insoluble in glycerol |
| Refractive index (nD) |
1.5681 (21 °C) |
| Pharmacology | |
| P03AX01 (WHO) QP53AX11 (WHO) | |
| Hazards | |
| EU classification (DSD) |
Harmful (Xn) |
| NFPA 704 | |
| Flash point | 158 °C (316 °F) (closed cup) |
| 481 °C (898 °F; 754 K) | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1700 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Benzyl benzoate is an organic compound with the formula C6H5CH2O2CC6H5. It is the ester of benzyl alcohol and benzoic acid. It forms either a viscous liquid or solid flakes and has a weak, sweet-balsamic odor. It occurs in a number of blossoms (e. g. tuberose, hyacinth) and is a component of Balsam of Peru and Tolu balsam.[1][2]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[3]
Uses
Benzyl benzoate is used as a topical acaricide, scabicide, and pediculicide in veterinary hospitals. It is also a repellent for chiggers, ticks, and mosquitoes.[4] It is an effective and inexpensive topical treatment for human scabies.[5] It has vasodilating and spasmolytic effects and is present in many asthma and whooping cough drugs.[6]
Benzyl benzoate is also used as a dye carrier, solvent for cellulose derivatives, plasticizer, and fixative in the perfume industry.[1]
Benzyl benzoate is an excipient in some testosterone-replacement medications (like Nebido) for treating hypogonadism.[7]
Side effects
Benzyl benzoate has low acute toxicity in laboratory animals. It is rapidly hydrolyzed to benzoic acid and benzyl alcohol. Benzyl alcohol is subsequently metabolized to benzoic acid. The conjugates of benzoic acid are rapidly eliminated in urine. When given in large doses to laboratory animals, benzyl benzoate can cause hyperexcitation, loss of coordination, ataxia, convulsions, and respiratory paralysis.[4]
Benzyl benzoate can be a skin irritant when used as a topical scabicide.[5] Overdose can result in blistering and hives or a rash can occur as an allergic reaction.[8][9]
As an excipient in some testosterone-replacement injectable medications, benzyl benzoate has been reported as a cause of anaphylaxis in a case in Australia.[10] Bayer includes this report in information for health professionals and recommends that physicians "should be aware of the potential for serious allergic reactions" to preparations of this type.[7] In Australia, reports to ADRAC, which evaluates reports of adverse drug reactions for the Therapeutic Goods Administration, show several reports of allergic issues since the anaphylaxis case from 2011.
Production
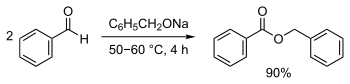
Benzyl benzoate is produced industrially by the reaction of sodium benzoate with benzyl alcohol in the presence of a base, or by transesterification of methyl benzoate and benzyl alcohol.[6] It is a byproduct of benzoic acid synthesis by toluene oxidation.[1] It can also be synthesized by the Tishchenko reaction, using benzaldehyde with sodium benzylate (generated from sodium and benzyl alcohol) as catalyst:[11][12]
References
- 1 2 3 Takao Maki; et al. (2007), "Benzoic Acid and Derivatives", Ullman's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 6
- ↑ Karl-Georg Fahlbusch; et al. (2007), "Flavors and Fragrances", Ullman's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 59
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 Jamaluddin Shaikh (2005), "Benzyl Benzoate", in Philip Wexler, Encyclopedia of Toxicology, 1 (2nd ed.), Elsevier, pp. 264–265
- 1 2 D.A. Burns (2010), "Diseases Caused by Arthropods and Other Noxious Animals", in Tony Burns; et al., Rook's Textbook of Dermatology, 2 (8th ed.), Wiley-Blackwell, p. 38.41
- 1 2 Friedrich Brühne, Elaine Wright (2007), "Benzyl Alcohol", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 7
- 1 2 "Nebido Monograph – Information for Health Care Professionls". Bayer. 2016 (updated). Retrieved 19 October 2016. Check date values in:
|date=(help) - ↑ "Benzyl Benzoate (Topical route)". PubMed Health at the United States National Library of Medicine. October 1, 2016. Retrieved October 19, 2016.
- ↑ "Benzocaine-Benzyl Benzoate Topical: Side Effects". WebMD. 2016. Retrieved October 19, 2016.
- ↑ Ong, G. S. Y.; Somerville, C. P.; Jones, T. W.; Walsh, J. P. (2012). "Anaphylaxis Triggered by Benzyl Benzoate in a Preparation of Depot Testosterone Undecanoate". Case Rep Med. 2012. doi:10.1155/2012/384054. PMC 3261473
 . PMID 22272209. 384054.
. PMID 22272209. 384054. - ↑ Friedrich Brühne, Elaine Wright (2007), "Benzaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 3
- ↑ Kamm, O.; Kamm, W. F. (1941). "Benzyl benzoate". Org. Synth.; Coll. Vol., 1, p. 104