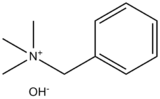
Benzyltrimethylammonium hydroxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Benzyl(trimethyl)azanium hydroxide | |
| Other names
Triton B, Trimethylbenzylammonium hydroxide, N,N,N-Trimethyl-1-phenylmethanaminium hydroxide | |
| Identifiers | |
| 100-85-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 60218 |
| ECHA InfoCard | 100.002.632 |
| PubChem | 66854 |
| UNII | 8P4488425W |
| |
| |
| Properties | |
| C10H17NO | |
| Molar mass | 167.25 g·mol−1 |
| Appearance | Liquid, clear, slightly yellow |
| Density | 0.95 g/mL |
| Miscible in water | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Benzyltrimethylammonium hydroxide, also known as Triton B or trimethylbenzylammonium hydroxide, is a quaternary ammonium salt that functions as an organic base. It is usually handled as a solution in water or methanol. The compound is colourless, although the solutions often appear yellowish.[1] Commercial samples often have a distinctive fish-like odour, presumably due to the presence of trimethylamine via hydrolysis.
Uses
Together with benzyltriethylammonium salt, benzyltrimethylammonium hydroxide is a popular phase-transfer catalyst.[2]
It is used in aldol condensation reactions and base-catalyzed dehydration reactions. It is also used as a base in Ando's Z-selective variant of Horner-Wadsworth-Emmons Olefination reactions.[3]
References
- ↑ Mary Ellen Bos "Benzyltrimethylammonium Hydroxide" in Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley & Sons. doi:10.1002/047084289X.rb079
- ↑ Marc Halpern "Phase-Transfer Catalysis" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_293
- ↑ Chaturvedi, D., & Ray, S. (2006). Triton b catalyzed, efficient, one-pot synthesis of carbamate esters from alcoholic tosylates. Monatshefte fuer Chemie, 137. Retrieved from http://www.springerlink.com/content/m48703268m3qw323/fulltext.pdf doi:10.1007/s00706-005-0452-2
See also
This article is issued from Wikipedia - version of the 10/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.