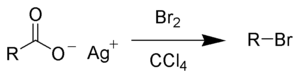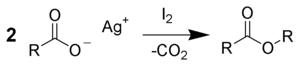Hunsdiecker reaction
| Hunsdiecker reaction | |
|---|---|
| Named after | Heinz Hunsdiecker Cläre Hunsdiecker |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | hunsdiecker-reaction |
| RSC ontology ID | RXNO:0000106 |
The Hunsdiecker reaction (also called the Borodin reaction after Alexander Borodin) is the organic reaction of silver salts of carboxylic acids with halogens to give organic halides.[1][2][3][4] It is an example of a halogenation reaction. The reaction is named after Heinz Hunsdiecker and Cläre Hunsdiecker, but was first noted by Borodin in 1861 when he prepared methyl bromide from silver acetate.[5]
Several reviews have been published.[6][7]
Mercuric oxide will also effect this transformation.[8][9]
Reaction mechanism
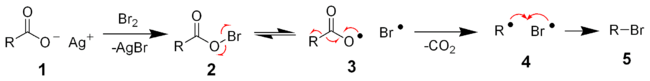
The reaction mechanism of the Hunsdiecker reaction is believed to involve organic radical intermediates. The silver salt of the carboxylic acid 1 will quickly react with bromine to form the acyl hypohalite intermediate 2. Formation of the diradical pair 3 allows for radical decarboxylation to form the diradical pair 4, which will quickly recombine to form the desired organic halide 5. The yield of halide is primary>secondary>tertiary.
Variations
Simonini reaction
The reaction of silver salts of carboxylic acids with iodine is called the Simonini reaction, named after Angelo Simonini, a student of Adolf Lieben at the University of Vienna. The ratio of the reagents play an important role in the determination of products, namely, if 1:1 ratio of salt and iodine is used alkyl iodide is formed. On the other hand, a 2:1 ratio gives RCOOR. and 3:2 ratio gives both the products.[6][10][11]
See also
References
- ↑ Cläre Hunsdiecker, et al. U.S. Patent # 2,176,181.
- ↑ Heinz Hunsdiecker; Cläre Hunsdiecker (1942). "Über den Abbau der Salze aliphatischer Säuren durch Brom". Ber. 75 (3): 291–297. doi:10.1002/cber.19420750309.
- ↑ Borodin, A. (1861). "Ueber Bromvaleriansäure und Brombuttersäure". Ann. 119: 121–123. doi:10.1002/jlac.18611190113.
- ↑ Allen, C. F. H.; Wilson, C. V. (1955). "Methyl 5-bromovalerate". Org. Synth.; Coll. Vol., 3, p. 578
- ↑ Jack Jie Li. Name Reactions: A Collection of Detailed Mechanisms and Synthetic ... Springer Science & Business Media. p. 328.
- 1 2 Johnson, R. G.; Ingham, R. K. (1956). "The Degradation of Carboxylic Acid Salts by Means of Halogen - the Hunsdiecker Reaction". Chem. Rev. 56 (2): 219–269. doi:10.1021/cr50008a002.
- ↑ Wilson, C. V. Org. React. 1957, 9, 341. (Review)
- ↑ Meek, J. S.; Osuga, D. T. (1973). "Bromocyclopropane". Org. Synth.; Coll. Vol., 5, p. 126
- ↑ Lampman, G. M.; Aumiller, J. C. (1988). "Mercury(II) oxide-modified Hunsdiecker reaction: 1-Bromo-3-chlorocyclobutane". Org. Synth.; Coll. Vol., 6, p. 179
- ↑ Simonini, A. (1892). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen". Monatshefte für Chemie. 13 (1): 320–325. doi:10.1007/BF01523646.
- ↑ Simonini, A. (1893). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen". Monatshefte für Chemie. 14 (1): 81–92. doi:10.1007/BF01517859.