C1 domain
| Phorbol esters/diacylglycerol binding domain (C1 domain) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
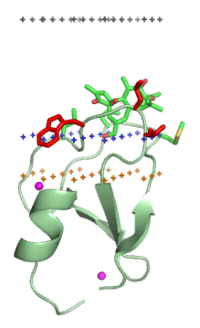
C1 domain of PKC-delta (1ptr)
Middle plane of the lipid bilayer - black dots. Boundary of the hydrocarbon core region - blue dots (cytoplasmic side). Layer of lipid phosphates - yellow dots. | |||||||||
| Identifiers | |||||||||
| Symbol | C1 | ||||||||
| Pfam | PF00130 | ||||||||
| InterPro | IPR002219 | ||||||||
| SMART | C1 | ||||||||
| PROSITE | PDOC00379 | ||||||||
| SCOP | 2cpk | ||||||||
| SUPERFAMILY | 2cpk | ||||||||
| OPM superfamily | 63 | ||||||||
| OPM protein | 1ptr | ||||||||
| CDD | cd00029 | ||||||||
| |||||||||
C1 domain (also known as phorbol esters/diacylglycerol binding domain) binds an important secondary messenger diacylglycerol (DAG), as well as the analogous phorbol esters.[1] Phorbol esters can directly stimulate protein kinase C, PKC. The N-terminal region of PKC, known as C1, has been shown[2]
Phorbol esters (such as PMA) are analogues of DAG and potent tumor promoters that cause a variety of physiological changes when administered to both cells and tissues. DAG activates a family of serine/threonine protein kinases, collectively known as protein kinase C (PKC). Phorbol esters can directly stimulate PKC.
The N-terminal region of PKC, known as C1, binds PMA and DAG in a phospholipid and zinc-dependent fashion. The C1 region contains one or two copies of a cysteine-rich domain, which is about 50 amino-acid residues long, and which is essential for DAG/PMA-binding.
The DAG/PMA-binding domain binds two zinc ions; the ligands of these metal ions are probably the six cysteines and two histidines that are conserved in this domain.
Human proteins containing this domain
AKAP13; ARAF; ARHGAP29; ARHGEF2; BRAF; CDC42BPA; CDC42BPB; CDC42BPG; CHN1; CHN2; CIT; DGKA; DGKB; DGKD; DGKE; DGKG; DGKH; DGKI; DGKK; DGKQ; DGKZ; GMIP; HMHA1; KSR1; KSR2; MYO9A; MYO9B; PDZD8; PRKCA; PRKCB1; PRKCD; PRKCE; PRKCG; PRKCH; PRKCI; PRKCN; PRKCQ; PRKCZ; PRKD1; PRKD2; PRKD3; RACGAP1; RAF1; RASGRP; RASGRP1; RASGRP2; RASGRP3; RASGRP4; RASSF1; RASSF5; ROCK1; ROCK2; STAC; STAC2; STAC3; TENC1; UNC13A; UNC13B; UNC13C; VAV1; VAV2; VAV3;
References
- ↑ Azzi A, Boscoboinik D, Hensey C (1992). "The protein kinase C family". Eur. J. Biochem. 208 (3): 547–557. doi:10.1111/j.1432-1033.1992.tb17219.x. PMID 1396661.
- ↑ Kikkawa U, Nishizuka Y, Igarashi K, Fujii T, Ono Y, Kuno T, Tanaka C (1989). "Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence". Proc. Natl. Acad. Sci. U.S.A. 86 (13): 4868–4871. doi:10.1073/pnas.86.13.4868. PMC 297516
 . PMID 2500657.
. PMID 2500657.
External links
- UMich Orientation of Proteins in Membranes families/superfamily-63 - Orientations of C1 domains in membranes (OPM)