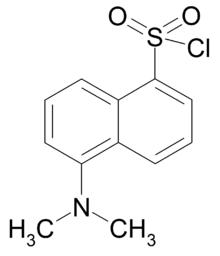
Dansyl chloride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5-(dimethylamino)naphthalene-1-sulfonyl chloride | |
| Identifiers | |
| 605-65-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:51907 |
| ChemSpider | 11308 |
| ECHA InfoCard | 100.009.175 |
| PubChem | 11801 |
| RTECS number | QK3688000 |
| |
| |
| Properties | |
| C12H12ClNO2S | |
| Molar mass | 269.74 g·mol−1 |
| Melting point | 70 °C (158 °F; 343 K) |
| Hazards | |
| EU classification (DSD) |
Corrosive (C) |
| R-phrases | R34 |
| S-phrases | S26 S36/37/39 S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dansyl chloride or 5-(DimethylAmino)Naphthalene-1-SulfonYL chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. It can also be made to react with secondary amines. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing and amino acid analysis.[2][3] It can also be used to label hydroxyl and carboxylic acid functional groups.[4]
Dansyl chloride may also be denoted DNSC. Likewise, a similar derivative, dansyl amide is known as DNSA.
In addition, these protein-DNSC conjugates are sensitive to their immediate environment. This, in combination with their ability to accept energy (as in fluorescence resonance energy transfer) from the amino acid tryptophan, allows this labeling technique to be used in investigating protein folding and dynamics.
The fluorescence of these sulfonamide adducts can be enhanced by adding alpha-cyclodextrin.[5] Dansyl chloride is unstable in dimethyl sulfoxide, which should never be used to prepare solutions of the reagent.[6]
The extinction coefficient of dansyl derivatives are important for measuring their concentration in solution. Dansyl chloride is one of the simplest sulfonamide derivatives, so it commonly serves as a starting reagent for the production of other derivatives. Exotic derivatives may have very different extinction coefficients, but others, such as dansyl amide, are similar to dansyl chloride in absorption and fluorescence characteristics. But even for dansyl chloride, there are a variety of extinction coefficient values that have been reported. Some of the values are used to estimate the extent of success in attempts to conjugate the dye to a protein. Other values may be used to determine a precise concentration of a stock solution. See the table below for specific values and their uses.
For all of the studies below, the absorption value is always taken at the maximum that appears between 310 nm and 350 nm. The peak is broad, so the measurement is not very sensitive. However, trivial errors due to wavelength calibration of the spectrophotometer can be avoided by taking the value at the maximum instead of strictly using 330 nm.
| Species | Extinction Coefficient [M−1·cm−1] | Notes |
|---|---|---|
| DNSC-protein | 3300[7] | Use for DNSC-protein conjugates; Used to determine degree of labeling in chymotrypsin and ovalbumin |
| DNSC | 4350[8] | In bicarbonate buffer; maximum is shifted to ~315 nm |
| DNSC | 4550[9] | In water; peak shifted to 312 nm |
| DNSA | 4050[9] | In 60% ethanol; Measured at 329 nm |
| DNSC | 4000[10] | Conditions are not given; no reference to the source of this value |
Preparation
This compound may be prepared by reacting the corresponding sulfonic acid with excess phosphorus oxychloride (POCl3) at room temperature.[11]
References
- ↑ "MSDS". Sigma-Aldrich. Retrieved 2007-12-02.
- ↑ Walker JM (1994). "Basic Protein and Peptide Protocols". Methods Mol. Biol. 32: 321–8. doi:10.1385/0-89603-268-X:321. ISBN 0-89603-268-X. PMID 7951732.
|chapter=ignored (help) - ↑ Walker JM (1994). "Basic Protein and Peptide Protocols". Methods Mol. Biol. 32: 329–34. doi:10.1385/0-89603-268-X:329. ISBN 0-89603-268-X. PMID 7951733.
|chapter=ignored (help) - ↑ Bartzatt R (2001). "Dansylation of hydroxyl and carboxylic acid functional groups". J. Biochem. Biophys. Methods. 47 (3): 189–195. doi:10.1016/S0165-022X(00)00136-6. PMID 11245890.
- ↑ Kinoshita T, Iinuma F, Tsuji A (1974). "Microanalysis of proteins and peptides. I. Enhancement of the fluorescence intensity of dansyl amino acids and dansyl proteins in aqueous media and its application to assay of amino acids and proteins". Chem. Pharm. Bull. 22 (10): 2413–20. doi:10.1248/cpb.22.2413. PMID 4468087.
- ↑ R. E. Boyle: The Reaction of Dimethyl Sulfoxide and 5-Dimethylaminonaphthalene-1-sulfonyl Chloride. In: The Journal of Organic Chemistry 31, Nr. 11, 1966, S. 3880- 3882, doi:10.1021/jo01349a529.
- ↑ Hartley, BS; V Massey (1956). "The active center of chymotrypsin: 1. Labelling with a fluorescent dye". Biochimica et Biophysica Acta. 21 (1): 58–70. doi:10.1016/0006-3002(56)90093-2. PMID 13363860.
- ↑ Chen, RF (1968). "Dansyl labeled proteins". Analytical Biochemistry. 25 (1): 412–416. doi:10.1016/0003-2697(68)90116-4. PMID 5704757.
- 1 2 Weber, G (1952). "Polarized fluorescence of Protein Conjugates". Biochemical journal. 51 (2): 145–155. PMC 1197814
 . PMID 14944566.
. PMID 14944566. - ↑ "Molecular Probes Handbook: Coumarins, Pyrenes and Other Ultraviolet Light–Excitable Fluorophores (Section 1.7)". Retrieved 12 June 2009.
- ↑ Arthur Mendel (1970). "Improved preparation of 5-dimethylamino-1-naphthalenesulfonyl chloride". J. Chem. Eng. Data. 15 (2): 340–341. doi:10.1021/je60045a010.