Desacetoxyvindoline
 | |
| Names | |
|---|---|
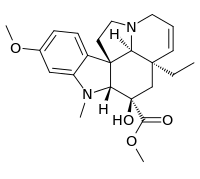
| IUPAC name
Methyl (2β,3β,5α,12β,19α)-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3-carboxylate | |
| Other names
Deacetoxyvindoline | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 388838 |
| PubChem | 439783 |
| |
| |
| Properties | |
| C23H30N2O4 | |
| Molar mass | 398.50 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Desacetoxyvindoline is a terpene idole alkaloid produced by the plant Catharanthus roseus. Desacetoxyvindoline is a product formed by the methylation of the nitrogen on the indole ring by the enzyme 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT).[1] The metabolite is a substrate for desacetoxyvindoline 4-hydroxylase (D4H) which catalyzes a hydroxylation to yield deacetylvindoline.[2]
References
- ↑ Liscombe, Usera and O’connor (2010) Homolog of tocopherol C methyltransferases catalyzes N methylation in anticancer alkaloid biosynthesis. Proceedings of the National Academy of Sciences. 107(44). 18793-18798
- ↑ Vazquez-Flota, De Carolis, Alarco and De Luca (1997) Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Molecular Biology. 34(6). 935-948
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.