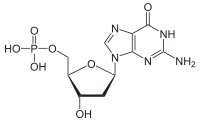
Deoxyguanosine monophosphate
 | |
 | |
| Identifiers | |
|---|---|
| 902-04-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16192 |
| ChemSpider | 58570 |
| ECHA InfoCard | 100.011.808 |
| 5122 | |
| PubChem | 65059 |
| |
| |
| Properties | |
| C10H14N5O7P | |
| Molar mass | 347.2243 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Deoxyguanosine monophosphate (dGMP), also known as deoxyguanylic acid or deoxyguanylate in its conjugate acid and conjugate base forms, respectively, is a derivative of the common nucleic acid guanosine triphosphate (GTP), in which the –OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose has been reduced to just a hydrogen atom (hence the "deoxy-" part of the name). It is used as a monomer in DNA.[1]
References
- ↑ Müller, Sabine (2008-09-08). Nucleic Acids from A to Z. John Wiley & Sons. ISBN 9783527622535.
See also
- Nucleic acid
- DNA metabolism
- Cofactor
- Guanosine
This article is issued from Wikipedia - version of the 11/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.