Diamondoid
In chemistry, diamondoids are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule.[1] These species are of interest as molecular approximations of the cubic diamond framework, terminated with C-H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately 5.6 * 10−22 grams.[2]
Examples
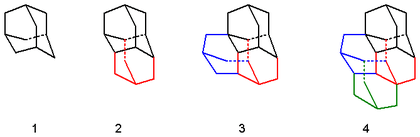
Examples include:
- Adamantane (C10H16)
- Iceane (C12H18)
- BC-8 (C14H20)
- Diamantane (C14H20) also diadamantane, two face-fused cages
- Triamantane (C18H24), also triadamantane. Diamantane has 4 identical faces available for anchoring a new C4H4 unit.
- Isotetramantane (C22H28). Triamantane has 8 faces on to which a new C4H4 unit can be added resulting in 4 isomers. One of these isomers displays a helical twist and is therefore prochiral. The P and M enantiomers have been separated.
- Pentamantane has 9 isomers with chemical formula C26H32 and one more pentamantane exists with chemical formula C25H30
- Cyclohexamantane (C26H30)
- Super-adamantane (C30H36)
- Basic beryllium acetate Be4O(O2CCH3)6
One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis using a keto-carbenoid reaction to attach cyclopentane rings.[3] Longer diamondoids have been formed from diamantane dicarboxylic acid.[4] The first ever isolation of a wide range of diamondoids from petroleum took place in the following steps:[1] a vacuum distillation above 345 °C, the equivalent atmospheric boiling point, then pyrolysis at 400 to 450 °C in order to remove all non-diamondoid compounds[5] and then a series of HPLC separation techniques.
In one study a tetramantane compound is fitted with thiol groups at the bridgehead positions.[6] This allows their anchorage to a gold surface and formation of self-assembled monolayers (diamond-on-gold). Additionally, functionalized diamondoids (adamantine) have been proposed as molecular building blocks for self-assembled molecular crystals.[7][8]
Organic chemistry of diamondoids even extends to pentamantane.[9] The medial position (base) in this molecule (the isomer [1(2,3)4]pentamantane) is calculated to yield a more favorable carbocation than the apical position (top) and simple bromination of pentamane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water / DMF forms the alcohol 3.

In contrast nitroxylation of 1 with nitric acid gives the apical nitrate 4 as an intermediate which is hydrolyzed to the apical alcohol 5 due to the higher steric demand of the active electrophilic NO2 - HNO3+ species. This alcohol can react with thionyl bromide to the bromide 6 and in a series of steps (not shown) to the corresponding thiol. Pentamantane can also react with tetrabromomethane and tetra-n-butylammonium Bromide (TBABr) in a free radical reaction to the bromide but without selectivity.
Origin and occurrence of diamondoids
Diamondoids are found in mature high-temperature petroleum fluids (volatile oils, condensates and wet gases). These fluids can have up to a spoonful of diamondoids per gallon (about 3.78 liters). A review by Mello and Moldowan in 2005 showed that although the carbon in diamonds is not biological in origin, the diamondoids found in petroleum are composed of carbon from biological sources. This was determined by comparing the ratios of carbon isotopes present.[10]
Optical and electronic properties
The optical absorption for all diamondoids lies deep in the UV spectral region with optical band gaps around 6 eV and higher.[11] The spectrum of each diamondoid is found to reflect its individual size, shape and symmetry. Due to their well defined size and structure diamondoids also serve as a model system for electronic structure calculations.[12] Many of the optoelectronic properties of diamondoids are determined by the difference in the nature of the highest occupied and lowest unoccupied molecular orbitals: the former is a bulk state, whereas the latter is a surface state. As a result, the energy of the lowest unoccupied molecular orbital is roughly independent of the size of the diamondoid.[13] [14]
Diamondoids have been found to exhibit a negative electron affinity, making them potentially useful in electron-emission devices.[13][15]
Nanotechnology
In the context of hypothetical building materials for nanotechnology components, "diamondoid" was used by K. Eric Drexler to refer to structures that would resemble diamond in a broad sense: strong, stiff structures containing dense, 3-D networks of covalent bonds, formed chiefly from first and second row atoms with a valence of three or more. Examples would include crystalline diamond, sapphire, and other stiff structures similar to diamond but with various atom substitutions which might include N, O, Si, S, and so forth.[16]
See also
- Other diamond-like compounds: Boron nitride
- Abiogenic petroleum origin
References
- 1 2 Dahl, J. E.; Liu, S. G.; Carlson, R. M. K. (3 January 2003). "Isolation and Structure of Higher Diamondoids, Nanometer-Sized Diamond Molecules". Science. 299 (5603): 96–99. doi:10.1126/science.1078239. PMID 12459548.
- ↑ J. E. P. Dahl; J. M. Moldowan; T. M. Peakman; J. C. Clardy; E. Lobkovsky; M. M. Olmstead; P. W. May; T. J. Davis; J. W. Steeds; K. E. Peters; A. Pepper; A. Ekuan; R. M. K. Carlson (2003). "Isolation and Structural Proof of the Large Diamond Molecule, Cyclohexamantane (C26H30)". Angewandte Chemie International Edition. 42 (18): 2040–2044. doi:10.1002/anie.200250794. PMID 12746817.
- ↑ Burns W, McKervey MA, Mitchell TR, Rooney JJ (1978). "A New Approach to the Construction of Diamondoid Hydrocarbons. Synthesis of anti-Tetramantane". JACS. 100 (3): 906–911. doi:10.1021/ja00471a041.
- ↑ Zhang J1, Zhu Z, Feng Y, Ishiwata H, Miyata Y, Kitaura R, Dahl JE, Carlson RM, Fokina NA, Schreiner PR, Tománek D, Shinohara H (Mar 25, 2013). "Evidence of diamond nanowires formed inside carbon nanotubes from diamantane dicarboxylic acid". Angewandte Chemie International Edition Engl. 52 (13): 3717–21. doi:10.1002/anie.201209192. PMID 23418054.
- ↑ Diamondoids are thermodynamically very stable and will survive this pyrolysis
- ↑ Functionalized Nanodiamonds Part 3: Thiolation of Tertiary/Bridgehead Alcohols Boryslav A. Tkachenko, Natalie A. Fokina, Lesya V. Chernish, Jeremy E. P. Dahl, Shenggao Liu, Robert M. K. Carlson, Andrey A. Fokin, and Peter R. Schreiner Org. Lett.; 2006; 8(9) pp 1767 - 1770; (Letter) Graphical abstract
- ↑ Markle, R. C. (2000). "Molecular building blocks and development strategies for molecular nanotechnology". Nanotechnology. 11: 89. Bibcode:2000Nanot..11...89M. doi:10.1088/0957-4484/11/2/309.
- ↑ Garcia, J. C.; Justo, J. F.; Machado, W. V. M.; Assali, L. V. C. (2009). "Functionalized adamantane: building blocks for nanostructure self-assembly". Phys. Rev. B. 80: 125421. arXiv:1204.2884
 . Bibcode:2009PhRvB..80l5421G. doi:10.1103/PhysRevB.80.125421.
. Bibcode:2009PhRvB..80l5421G. doi:10.1103/PhysRevB.80.125421. - ↑ Fokin, Andrey A.; Schreiner, Peter R.; Fokina, Natalie A.; Tkachenko, Boryslav A.; Hausmann, Heike; Serafin, Michael; Dahl, Jeremy E. P.; Liu, Shenggao; Carlson, Robert M. K. (2006). "Reactivity of [1(2,3)4]Pentamantane (Td-Pentamantane): A Nanoscale Model of Diamond". J. Org. Chem. 71 (22): 8532–8540. doi:10.1021/jo061561x.
- ↑ Petroleum: To Be Or Not To Be Abiogenic, by M. R. Mello and J. M. Moldowan; #90043 (2005)
- ↑ L. Landt, K.Klünder; J. E. Dahl; R. M. K. Carlson; T. Möller & C. Bostedt (2009). "Optical Response of Diamond Nanocrystals as a Function of Particle Size, Shape, and Symmetry". Phys. Rev. Lett. 103: 047402. Bibcode:2009PhRvL.103d7402L. doi:10.1103/PhysRevLett.103.047402.
- ↑ M. Vörös; A. Gali (2009). "Optical absorption of diamond nanocrystals from ab initio density-functional calculations". Phys. Rev. B. 80: 161411. Bibcode:2009PhRvB..80p1411V. doi:10.1103/PhysRevB.80.161411.
- 1 2 N. D. Drummond; A. J. Williamson; R. J. Needs; G. Galli (2005). "Electron emission from diamondoids: a diffusion quantum Monte Carlo study". Physical Review Letters. 95: 096801–096804. arXiv:0801.0381
 . Bibcode:2005PhRvL..95i6801D. doi:10.1103/PhysRevLett.95.096801.
. Bibcode:2005PhRvL..95i6801D. doi:10.1103/PhysRevLett.95.096801. - ↑ T. M. Willey; C. Bostedt; T. van Buuren; J. E. Dahl; S. G. Liu; R. M. K. Carlson; L. J. Terminello; T. Moller (2005). "Molecular Limits to the Quantum Confinement Model in Diamond Clusters". Physical Review Letters. 95: 113401–113404. Bibcode:2005PhRvL..95k3401W. doi:10.1103/PhysRevLett.95.113401.
- ↑ W. L. Yang; J. D. Fabbri; T. M. Willey; J. R. I. Lee; J. E. Dahl; R. M. K. Carlson; P. R. Schreiner; A. A. Fokin; B. A. Tkachenko; N. A. Fokina; W. Meevasana; N. Mannella; K. Tanaka; X. J. Zhou; T. van Buuren; M. A. Kelly; Z. Hussain; N. A. Melosh; Z.-X. Shen (2007). "Monochromatic Electron Photoemission from Diamondoid Monolayers". Science. 316: 1460–1462. Bibcode:2007Sci...316.1460Y. doi:10.1126/science.1141811.
- ↑ Drexler, Eric (1992). Nanosystems: Molecular Machinery, Manufacturing,and Computation. Wiley. ISBN 978-0471575184.
External links
- Cluster and Nanocrystal Research Group, Technische Universität Berlin
- Molecular Diamond Technologies, Chevron Texaco
- Nanotechnology and the arrival of the Diamond Age
- Laser Raman Spectroscopy and Modelling of Diamondoids
- Electronic and Optical Properties of Diamondoids (free download)
- Diamondoid Molecules: With Applications in Biomedicine, Materials Science, Nanotechnology & Petroleum Science