Dihydropteroate
 | |
| Names | |
|---|---|
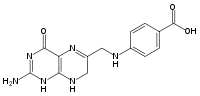
| IUPAC name
4-{[(2-amino-4-oxo-1,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoic acid | |
| Identifiers | |
| 2134-76-1 | |
| 3D model (Jmol) | Interactive image Interactive image |
| 1226443 | |
| ChEBI | CHEBI:4581 |
| ChemSpider | 165 |
| KEGG | C00921 |
| PubChem | 170 |
| |
| |
| Properties | |
| C14H14N6O3 | |
| Molar mass | 314.3 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dihydropteroate is a pterin created from para-aminobenzoic acid (PABA) by the enzyme dihydropteroate synthase. It is an important intermediate in folate synthesis.

Bacteriostatic agents, such as sulfonamides, target dihydropteroate synthetase. The effect of dihydropteroate synthetase inhibition is comparable to that of dihydrofolate reductase inhibition by trimethoprim, another bacteriostatic agent. Together these two drugs - trimethoprim-sulfamethoxazole [TMP-SMX] - are commonly used to treat recurrent urinary tract, Shigella, Salmonella, and Pneumocystis jivoreci infections.
See also
This article is issued from Wikipedia - version of the 11/30/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.