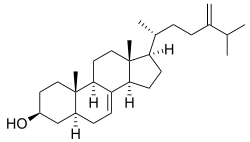
Episterol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3S,5S,10S,13R,14R,17R)-10,
13-dimethyl-17-[(2R)-6-methyl-5-methylideneheptan-2-yl]-2,3,4,5,6,9,11, 12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Systematic IUPAC name
(3β,5α)-Ergosta-7,24(28)-dien-3-ol | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:23929 |
| ChemSpider | 4446754 |
| MeSH | Episterol |
| PubChem | 5283662 |
| |
| |
| Properties | |
| C28H46O | |
| Molar mass | 398.66 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Episterol is a sterol involved in the biosynthesis of steroids. Episterol is converted from 24-methylenelophenol. Episterol is converted to 5-dehydroepisterol by the enzyme lathosterol oxidase. Episterol is also known to be a precursor to ergosterol.
External links
This article is issued from Wikipedia - version of the 8/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.