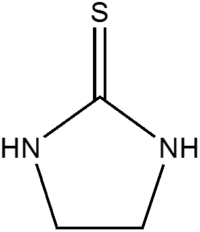
Ethylene thiourea
 | |
| Names | |
|---|---|
| IUPAC name
Imidazolidine-2-thione | |
| Other names
1,3-Ethylene-2-thiourea, N,N-Ethylenethiourea | |
| Identifiers | |
| 96-45-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 2005851 |
| ECHA InfoCard | 100.002.280 |
| PubChem | 2723650 |
| |
| |
| Properties | |
| C3H6N2S | |
| Molar mass | 102.16 g·mol−1 |
| Appearance | White to pale-green crystalline solid |
| Odor | Faint, amine-like |
| Melting point | 203 °C (397 °F; 476 K) |
| Boiling point | 347.18 °C (656.92 °F; 620.33 K) |
| 2% (30 °C)[1] | |
| Vapor pressure | 16 mmHg (20 °C)[1] |
| Hazards | |
| Main hazards | combustible[1] |
| Flash point | 252.2 °C (486.0 °F; 525.3 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1832 mg/kg (oral, rat)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
Ca Use encapsulated form.[1] |
| IDLH (Immediate danger) |
Ca [N.D.][1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethylene thiourea is an organosulfur compound. It is an example of an N,N-disubstituted thiourea.
This compound is synthesized by treating ethylenediamine with carbon disulfide.[3]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0276". National Institute for Occupational Safety and Health (NIOSH).
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/rn/96-45-7
- ↑ C. F. H. Allen; C. O. Edens; James VanAllan. "Ethylene Thiourea". Org. Synth.; Coll. Vol., 3, p. 394
This article is issued from Wikipedia - version of the 3/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.