Extracellular polymeric substance
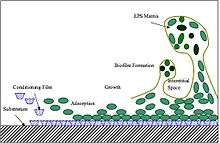
Extracellular polymeric substances (EPSs) are natural polymers of high molecular weight secreted by microorganisms into their environment.[1] EPSs establish the functional and structural integrity of biofilms, and are considered the fundamental component that determines the physiochemical properties of a biofilm.[2]
EPSs are mostly composed of polysaccharides (exopolysaccharides) and proteins, but include other macro-molecules such as DNA, lipids and humic substances. EPSs are the construction material of bacterial settlements and either remain attached to the cell's outer surface, or are secreted into its growth medium. These compounds are important in biofilm formation and cells attachment to surfaces. EPSs constitute 50% to 90% of a biofilm's total organic matter.[2][3][4]
Exopolysaccharides (also sometimes abbreviated EPSs) are high-molecular-weight polymers that are composed of sugar residues and are secreted by a microorganism into the surrounding environment. Microorganisms synthesize a wide spectrum of multifunctional polysaccharides including intracellular polysaccharides, structural polysaccharides and extracellular polysaccharides or exopolysaccharides. Exopolysaccharides generally consist of monosaccharides and some non-carbohydrate substituents (such as acetate, pyruvate, succinate, and phosphate). Owing to the wide diversity in composition, exopolysaccharides have found multifarious applications in various food and pharmaceutical industries. Many microbial EPSs provide properties that are almost identical to the gums currently in use. With innovative approaches, efforts are underway to supersede the traditionally used plant and algal gums by their microbial counterparts. Moreover, considerable progress has been made in discovering and developing new microbial EPSs that possess novel industrial significance.[5]
Function
The exopolysaccharides of some strains of lactic acid bacteria, e.g., Lactococcus lactis subsp. cremoris, contribute a gelatinous texture to fermented milk products (e.g., Viili), and these polysaccharides are also digestible.[6][7]
Capsular exopolysaccharides can protect pathogenic bacteria and contribute to their pathogenicity. Attachment of nitrogen-fixing bacteria to plant roots and soil particles, which is important for colonisation of rhizosphere and roots and for infection of the plant, can be mediated by exopolysaccharides as well. An example for industrial use of exopolysaccharides is the application of dextran in panettone and other breads in the bakery industry.[8] Exopolysaccharides also have an important role in endodontic infections.
List
.svg.png)
- acetan (Acetobacter xylinum)
- alginate (Azotobacter vinelandii)
- cellulose (Acetobacter xylinum)
- chitosan (Mucorales spp.)
- curdlan (Alcaligenes faecalis var. myxogenes)
- cyclosophorans (Agrobacterium spp., Rhizobium spp. and Xanthomonas spp.)
- dextran (Leuconostoc mesenteroides, Leuconostoc dextranicum and Lactobacillus hilgardii)
- emulsan (Acinetobacter calcoaceticus)
- galactoglucopolysaccharides (Achromobacter spp., Agrobacterium radiobacter, Pseudomonas marginalis, Rhizobium spp. and Zooglea' spp.)
- galactosaminogalactan (Aspergillus spp.)
- gellan (Aureomonas elodea and Sphingomonas paucimobilis)
- glucuronan (Sinorhizobium meliloti)
- N-acetylglucosamine (Staphylococcus epidermidis)
- N-acetyl-heparosan (Escherichia coli)
- hyaluronic acid (Streptococcus equi)
- indican (Beijerinckia indica)
- kefiran (Lactobacillus hilgardii)
- lentinan (Lentinus elodes)
- levan (Alcaligenes viscosus, Zymomonas mobilis, Bacillus subtilis)
- pullulan (Aureobasidium pullulans)
- scleroglucan (Sclerotium rolfsii, Sclerotium delfinii and Sclerotium glucanicum)
- schizophyllan (Schizophylum commune)
- stewartan (Pantoea stewartii subsp. stewartii)
- succinoglycan (Alcaligenes faecalis var myxogenes, Sinorhizobium meliloti)
- xanthan (Xanthomonas campestris)
- welan (Alcaligenes spp.)
See also
- Extracellular matrix in multi-cellular organisms
References
- ↑ Staudt C, Horn H, Hempel DC, Neu TR (2004). "Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms". Biotechnol. Bioeng. 88 (5): 585–92. doi:10.1002/bit.20241. PMID 15470707.
- 1 2 Flemming, Hans-Curt; Wingender, Jost; Griebe, Thomas; Mayer, Christian (December 21, 2000), "Physico-Chemical Properties of Biofilms", in L. V. Evans, Biofilms: Recent Advances in their Study and Control, CRC Press, p. 20, ISBN 978-9058230935
- ↑ Donlan RM (2002). "Biofilms: microbial life on surfaces". Emerging Infect. Dis. 8 (9): 881–90. doi:10.3201/eid0809.020063. PMC 2732559
 . PMID 12194761.
. PMID 12194761. - ↑ Donlan RM, Costerton JW (2002). "Biofilms: survival mechanisms of clinically relevant microorganisms". Clin. Microbiol. Rev. 15 (2): 167–93. doi:10.1128/CMR.15.2.167-193.2002. PMC 118068
 . PMID 11932229.
. PMID 11932229. - ↑ Suresh and Mody (2009). "Microbial Exopolysaccharides: Variety and Potential Applications". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
- ↑ Welman AD (2009). "Exploitation of Exopolysaccharides from lactic acid bacteria". Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.
- ↑ Ljungh A, Wadstrom T (editors) (2009). Lactobacillus Molecular Biology: From Genomics to Probiotics. Caister Academic Press. ISBN 978-1-904455-41-7.
- ↑ Ullrich M (editor) (2009). Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.