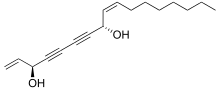
Falcarindiol
 | |
| Names | |
|---|---|
| IUPAC name
(3R,8S,9Z)-1,9-Heptadecadiene-4,6-diyne-3,8-diol | |
| Other names
cis-Heptadeca-1,9-diene-4,6-diyne-3,8-diol | |
| Identifiers | |
| 55297-87-5 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL69018 |
| ChemSpider | 4444588 |
| PubChem | 5281148 |
| |
| |
| Properties | |
| C17H24O2 | |
| Molar mass | 260.371 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Falcarindiol is a polyacetylene found in carrot roots which has antifungal activity.[1][2] Falcarindiol is the main compound responsible for bitterness in carrots.[3] Falcarindiol and other falcarindiol-type polyacetylenes are also found in many other plants of the Apiaceae family, including some commonly used seasonings such as dill and parsley.[4] A variety of bioactivities have been reported so far for falcaridiol and the falcarindiol-type polyacetylenes,[5][6] and because of potential health-promoting metabolic effects these compounds are studied as potential nutraceuticals.[7] It is the most-active among several polyynes with potential anticancer activity found in Devil's Club (Oplopanax horridus), a medicinal plant used by many indigenous peoples in Alaska and the Pacific Northwest.[8]
See also
References
- ↑ Garrod, B. (1978). "Cis-heptadeca-1,9-diene-4,6-diyne-3,8-diol, an antifungal polyacetylene from carrot root tissue". Physiologial Plant Pathology. 13 (2): 241–246. doi:10.1016/0048-4059(78)90039-5.
- ↑ Kemp, M. S. (1978). "Falcarindiol: An antifungal polyacetylene from Aegopodium podagraria". Phytochemistry. 17 (5): 1002. doi:10.1016/S0031-9422(00)88669-0.
- ↑ Czepa, A.; Hofmann, T. (2003). "Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree". Journal of Agricultural and Food Chemistry. 51 (13): 3865–3873. doi:10.1021/jf034085+. PMID 12797757.
- ↑ Christensen, L. P.; Brandt, K. (2006). "Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis". Journal of Pharmaceutical and Biomedical Analysis. 41 (3): 683–693. doi:10.1016/j.jpba.2006.01.057. PMID 16520011.
- ↑ Jin, H. R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C. Z.; Huang, W. H.; Li, S. P.; He, T. C.; Yuan, C. S.; Du, W. (2012). "The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress". Cell Death and Disease. 3 (8): e376. doi:10.1038/cddis.2012.122. PMC 3434669
 . PMID 22914324.
. PMID 22914324. - ↑ Wyrembek, P.; Negri, R.; Kaczor, P.; Czyżewska, M.; Appendino, G.; Mozrzymas, J. W. (2012). "Falcarindiol allosterically modulates GABAergic currents in cultured rat hippocampal neurons". Journal of Natural Products. 75 (4): 610–616. doi:10.1021/np2008522. PMID 22432736.
- ↑ Christensen, L. P. (2011). "Aliphatic C17-Polyacetylenes of the Falcarinol Type as Potential Health Promoting Compounds in Food Plants of the Apiaceae Family". Recent Patents on Food, Nutrition and Agriculture. 3 (1): 64–77. doi:10.2174/2212798411103010064. PMID 21114468.
- ↑ Sun, S; Du, GJ; Qi, LW; Williams, S; Wang, CZ; Yuan, CS (2010). "Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.)". J Ethnopharmacol. 132 (1): 280–285. doi:10.1016/j.jep.2010.08.026.