Fenestrane

A fenestrane in organic chemistry is a type of chemical compound with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles.[1] They can be regarded as spiro compounds twice over. Because of their inherent strain and instability, fenestranes are of theoretical interest to chemists. The name—proposed in 1972 by Vlasios Georgian and Martin Saltzman[2]—is derived from the Latin word for window, fenestra.
The smallest member of the family, consisting of four fused cyclopropane rings is [3.3.3.3]fenestrane or pyramidane—a molecule with an extensive history on its own. In the next member, four cyclobutane rings are fused, forming the archetypical window motif. It is called in its own chemical nomenclature [4.4.4.4]fenestrane simply by counting the number of carbon atoms in each ring. The formal name for this compound is tetracyclo[3.3.1.03,9.07,9]nonane.
In an extreme case the central carbon atom ordinarily with a tetrahedral molecular geometry gets completely flattened. In the molecular orbital picture for square planar methane two of a total of three sp2-hybridized carbon atomic orbitals form regular bonds with two of the hydrogen atoms as in a planar alkene. The third sp2-orbital interacts in a three-center two-electron bond with the two remaining hydrogen atoms utilizing only the hydrogen electrons. Two additional carbon valence electrons are situated in a p-orbital perpendicular to the plane of the molecule. The four C–H bonds are equal because they resonate. In silico calculations show that it takes 95 to 250 kcal/mol (400 to 1,050 kJ/mol) for this process.
One of the highest strained fenestranes actually isolated is a [4.4.4.5]fenestrane with bond angles through the central carbon atom of around 130° based on X-ray diffraction. In this molecule the bonds extending from the central carbon atom are shortened with bond lengths of 149 pm while those at the perimeter are extended to 159 pm. (The C–C bond in ethane is 155 pm long.)[3]
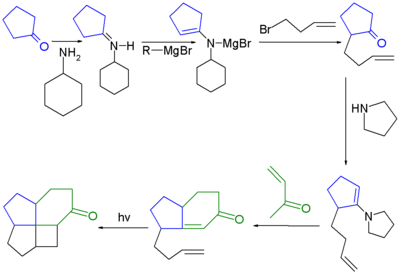
The first ever synthesized fenestrane was a [4.5.5.6]fenestrane:[2][4]
Pyramidanes
Pyramidane ([3.3.3.3]fenestrane) is a molecule related to tetrahedrane with formal name tetracyclo-[2.1.0.01,3.02,5]pentane. The compound is also related to spiropentadiene that has a similar quaternary carbon atom. The compound has never been synthesised. The synthesis of related germa- and stannapyramidanes Ge[C4(SiMe3)4] and Sn[C4(SiMe3)4] on the other hand has been reported.[5]
Exploits
In one study a [4.5.5.5]fenestrane was synthesized with one carbon atom replaced by nitrogen because aza- compounds and their salts are more likely to form crystalline compounds suitable for X-ray analysis.[6] In step 1 the alkyl halide 1-iodo-3-butene 1 is converted to a cyanozinc cuprate 2 (by transmetalation of the organozinc iodide with copper cyanide) which reacts in the next step with 1-nitrocyclopentene 3 in a nucleophilic addition whereby the nitronate 4 is captured by phenylselenenyl bromide to the selenium intermediate 5. Hydrogen peroxide oxidation of 5 yields the nitroalkene 6 as a mixture of syn and anti isomers. A [4+2]cycloaddition with n-butylenol ether in presence of trimethylaluminium gives the nitronate 7 and a second [3+2]cycloaddition by heating in presence of potassium carbonate gives the nitroso acetal 8. Hydrogenation with Raney nickel gives the diol 9 which on a double Mitsunobu reaction (with an amine proton donor) gives the azafenestrane 10 as the borane salt.

In the borane salt the N–C–C bond angle is 126°.
One study describes an unusual 8π disrotatory – 6π conrotatory electrocyclic cascade reaction aiming to minimise the number of steps required to synthesise a fenestrane.[7][8]

References
- ↑ Venepalli, Bhaskar Rao; Agosta, William C. (1987). "Fenestranes and the flattening of tetrahedral carbon". Chem. Rev. 87 (2): 399–410. doi:10.1021/cr00078a007.
- 1 2 Georgian, Vlasios; Saltzman, Martin (1972). "Syntheses directed toward saturated "flat" carbon". Tetrahedron Letters. 13 (42): 4315–4317. doi:10.1016/S0040-4039(01)94304-7.
- ↑ Rao, V. Bhaskar; George, Clifford F.; Wolff, Steven; Agosta, William C. (1985-10-01). "Synthetic and structural studies in the [4.4.4.5]fenestrane series". Journal of the American Chemical Society. 107 (20): 5732–5739. doi:10.1021/ja00306a022.
- ↑ The first step in this reaction sequence is an adaptation of the Stork enamine alkylation reacting cyclopentanone with 3-bromo-1-butene through an imine derivative with pyrrolidine and forming a magnesium salt with ethyl magnesium bromide. The next step is a regular Stork enamine reaction followed by an aldol condensation forming the cyclohexenone ring. The final step is a photolytic [2+2]cycloaddition.
- ↑ Lee, Vladimir Ya.; Ito, Yuki; Sekiguchi, Akira; Gornitzka, Heinz; Gapurenko, Olga A.; Minkin, Vladimir I.; Minyaev, Ruslan M. (2013). "Pyramidanes". J. Am. Ch. Soc. 135 (24): 8794–8797. doi:10.1021/ja403173e.
- ↑ Denmark, Scott E.; Montgomery, Justin I.; Kramps, Laurenz A. (2006). "Synthesis, X-ray Crystallography, and Computational Analysis of 1-Azafenestranes". J. Am. Chem. Soc. 128 (35): 11620–11630. doi:10.1021/ja0632759.
- ↑ Hulot, C.; Blond, G.; Suffert, J. (2008). "Synthesis of [4.6.4.6]Fenestradienes and [4.6.4.6]Fenestrenes Based on an 8π−6π-Cyclization-Oxidation Cascade". J. Am. Chem. Soc. 130 (15): 5046–5047. doi:10.1021/ja800691c.
- ↑ Reagents: P-2 Ni (Ni(OAc)2·4H2O) / hydrogen gas. Reaction initiated by organic reduction of alkyne to alkene