HHTDD
 | |
| Names | |
|---|---|
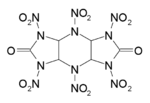
| IUPAC name
2,6-dioxo-1,3,4,5,7,8-hexanitrodecahydro-1H,5H-diimidazo[4,5-b:4',5'-e]pyrazine | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23078717 |
| |
| |
| Properties | |
| C6H4N12O14 | |
| Molar mass | 468.168 |
| Explosive data | |
| Detonation velocity | 9700 m/s |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
HHTDD is a powerful but moisture sensitive explosive compound. It is essentially an open analogue of the cyclic nitroamine cage compounds such as CL-20. While it is highly explosive, with a velocity of detonation even higher than that of CL-20, HHTDD readily decomposes in the presence of even trace amounts of water, making it unsuitable for any practical applications.[1]
See also
- 2,4,6-Tris(trinitromethyl)-1,3,5-triazine
- 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)
- Heptanitrocubane
- Octanitrocubane
- RE factor
References
- ↑ Vedachalam, Murugappa; Ramakrishnan, Vayalakkavoor T.; Boyer, Joseph H.; Dagley, Ian J.; Nelson, Keith A.; Adolph, Horst G.; Gilardi, Richard; George, Clifford; Flippen-Anderson, Judith L. (1991). "Facile synthesis and nitration of cis-syn-cis-2,6-dioxodecahydro-1H,5H-diimidazo[4,5-b:4',5'-e]pyrazine". The Journal of Organic Chemistry. 56 (10): 3413–3419. doi:10.1021/jo00010a043.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.