Hell–Volhard–Zelinsky halogenation
| Hell–Volhard–Zelinsky halogenation | |
|---|---|
| Named after | Carl Magnus von Hell Jacob Volhard Nikolay Zelinsky |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | hell-volhard-zelinsky-reaction |
The Hell–Volhard–Zelinsky halogenation reaction halogenates carboxylic acids at the α carbon. The reaction is named after three chemists, the German chemists Carl Magnus von Hell (1849–1926) and Jacob Volhard (1834–1910) and the Russian chemist Nikolay Zelinsky (1861–1953).[1][2][3][4]
Scheme
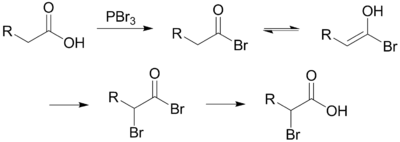
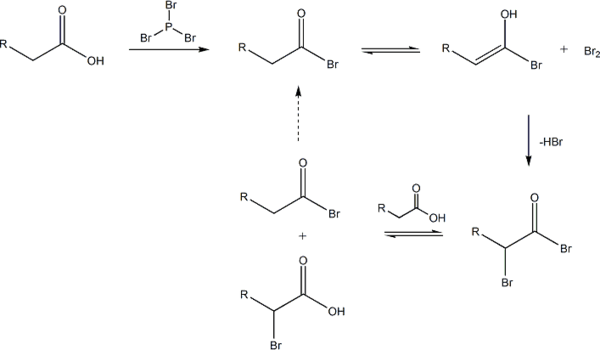
Unlike other halogenation reactions, this reaction takes place in the absence of a halogen carrier. The reaction is initiated by addition of a catalytic amount of PBr3, after which one molar equivalent of Br2 is added.
PBr3 replaces the carboxylic OH with a bromide, resulting in a carboxylic acid bromide. The acyl bromide can then tautomerize to an enol, which will readily react with the Br2 to brominate a second time at the α position.
In neutral to slightly acidic aqueous solution, hydrolysis of the α-bromo acyl bromide occurs spontaneously, yielding the α-bromo carboxylic acid in an example of a nucleophilic acyl substitution. If an aqueous solution is desirable, a full molar equivalent of PBr3 must be used as the catalytic chain is disrupted.
If little nucleophilic solvent is present, reaction of the α-bromo acyl bromide with the carboxylic acid yields the α-bromo carboxylic acid product and regenerates the acyl bromide intermediate. In practice a molar equivalent of PBr3 is often used anyway to overcome the slow reaction kinetics.
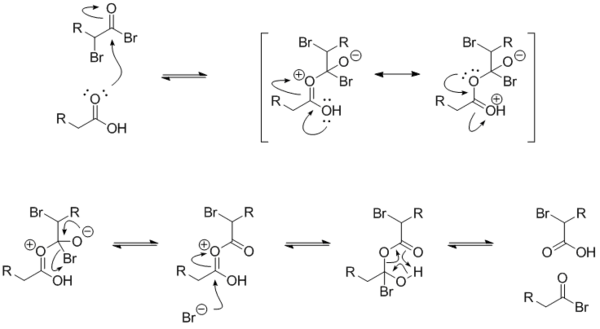
The mechanism for the exchange between an alkanoyl bromide and a carboxylic acid is below. The α-bromoalkanoyl bromide has a strongly electrophilic carbonyl carbon because of the electron-withdrawing effects of the two bromides.
See also
References
- ↑ Hell C. (1881). "Ueber eine neue Bromirungsmethode organischer Säuren". Berichte. 14: 891–893. doi:10.1002/cber.188101401187.
- ↑ Volhard J. (1887). "Ueber Darstellung α-bromirter Säuren". Annalen der Chemie. 242: 141–163. doi:10.1002/jlac.18872420107.
- ↑ Zelinsky N. (1887). "Ueber eine bequeme Darstellungsweise von α-Brompropionsäureester". Berichte. 20: 2026–2026. doi:10.1002/cber.188702001452.
- ↑ Allen C. F., Kalm M. J. (1963). "2-Methylenedodecanoic Acid". Org. Synth.; Coll. Vol., 4, p. 616