Hydroxylammonium nitrate
 | |
 | |
| Names | |
|---|---|
| Other names
hydroxylamine nitrate | |
| Identifiers | |
| 13465-08-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 24259 |
| ECHA InfoCard | 100.033.342 |
| EC Number | 236-691-2 |
| PubChem | 26045 |
| |
| |
| Properties | |
| H4N2O4 | |
| Molar mass | 96.04 g/mol |
| Density | 1.84 g/cm3 |
| Melting point | 48 °C |
| Soluble | |
| Hazards | |
| Safety data sheet | External MSDS (as 18 % solution) |
| EU classification (DSD) |
Explosive (E) Carc. Cat. 3 Toxic (T) Harmful (Xn) Irritant (Xi) Dangerous for the environment (N) |
| R-phrases | R2, R22, R24, R36/38, R40, R43, R48/22, R50 |
| S-phrases | (S1/2), S26, S36/37, S45, S61 |
| Related compounds | |
| Other anions |
Hydroxylammonium sulfate Hydroxylammonium chloride |
| Other cations |
Ammonium nitrate |
| Related compounds |
Hydroxylamine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
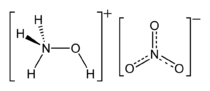
Hydroxylammonium nitrate or hydroxylamine nitrate (HAN) is an inorganic compound with the chemical formula NH3OHNO3. It is a salt derived from hydroxylamine and nitric acid. In its pure form, it is a colourless hygroscopic solid. It has potential to be used as a rocket propellant either as a solution in monopropellants or bipropellants.[1]
Properties
The compound is a salt with separated hydroxyammonium and nitrate ions.[2] Hydroxylammonium nitrate is unstable because it contains both a reducing agent (hydroxylammonium cation) and an oxidizer (nitrate),[3] the situation being analogous to ammonium nitrate. It is usually handled as an aqueous solution. The solution is corrosive and toxic, and may be carcinogenic. Solid HAN is unstable, particularly in the presence of trace amounts of metal salts.
Applications
HAN is a potential rocket propellant, both in the solid form as a solid propellant oxidizer, and in the aqueous solution in monopropellant rockets, including the Network Centric Airborne Defense Element boost-phase interceptor being developed by Raytheon.[4] It is typically bonded with glycidyl azide polymer (GAP), hydroxyl-terminated polybutadiene (HTPB), or carboxy-terminated polybutadiene (CTPB) and requires preheating to 200-300 °C to decompose. The catalyst is a noble metal, similar to the other monopropellants that use silver or palladium.
It will be used in a fuel/oxidizer blend known as "AF-M315E" in the high thrust engines of the Green Propellant Infusion Mission in 2017.[5][6][7] The specific impulse of AF-M315E is 257 s.[1]
HAN is sometimes used in nuclear reprocessing as a reducing agent for plutonium ions.
Bibliography
- Donald G. Harlow et al. (1998). "Technical Report on Hydroxlyamine Nitrate". U.S. Department of Energy. DOE/EH-0555
- Gösta Bengtsson et al. (2002) "The kinetics and mechanism of oxidation of hydroxylamine by iron(III)". J. Chem. Soc., Dalton Trans., 2002, 2548–2552
References
- 1 2 Spores, Ronald A.; Masse, Robert; Kimbrel, Scott; McLean, Chris (15–17 July 2013). "49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit" (PDF). San Jose, California, USA. Archived (PDF) from the original on 2014-02-28.
|contribution=ignored (help) - ↑ Rheingold, A. L.; Cronin, J. T.; Brill, T. B.; Ross, F. K. (March 1987). "Structure of hydroxylammonium nitrate (HAN) and the deuterium homolog". Acta Crystallographica Section C. 43 (3): 402–404. doi:10.1107/S0108270187095593.
- ↑ Pembridge, John R.; et al. (1979). Kinetics, Mechanism, and Stoicheiometry of the Oxidation of Hydroxylamine by Nitric Acid. JCS Dalton. pp. 1657–1663.
- ↑ "Boost phase interceptor". Press Releases. Raytheon. Archived from the original on May 18, 2007.
- ↑ "About Green Propellant Infusion Mission (GPIM)". NASA. 2014. Archived from the original on 2013-04-24.
|first1=missing|last1=in Authors list (help) - ↑ "Green Propellant Infusion Mission (GPIM)". Ball Aerospace. 2014. Archived from the original on 2013-04-24.
- ↑ Casey, Tina (19 July 2013). "NASA Sets Its Sights On $45 Million Green Fuel Mission". Clean Technica.