Ibritumomab tiuxetan
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Mouse |
| Target | CD20 |
| Clinical data | |
| Trade names | Zevalin |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | intravenous |
| ATC code | V10XX02 (WHO) (90Y) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
206181-63-7 |
| DrugBank |
DB00078 |
| ChemSpider | none |
| UNII |
4Q52C550XK |
| ChEMBL |
CHEMBL1201606 |
| | |
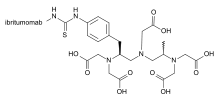
Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for relapsed or refractory, low grade or transformed B cell non-Hodgkin's lymphoma, a lymphoproliferative disorder. The drug uses the monoclonal mouse IgG1 antibody ibritumomab (pronounced as <ih bri TYOO mo mab>)[1] in conjunction with the chelator tiuxetan, to which a radioactive isotope (either yttrium-90 or indium-111) is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.[2][3]
Mechanism of action
The antibody binds to the CD20 antigen found on the surface of normal and malignant B cells (but not B cell precursors), allowing radiation from the attached isotope (mostly beta emission) to kill it and some nearby cells. In addition, the antibody itself may trigger cell death via antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and apoptosis. Together, these actions eliminate B cells from the body, allowing a new population of healthy B cells to develop from lymphoid stem cells.
Preparation
Zevalin is supplied as a single dosage kit supplied by IDEC Pharmaceuticals Corp. It consists of Ibritumomab covalently conjugated to the metal chelator tiuxetan, which forms a stable complex with indium-111 for imaging and yttrium-90 for therapy.
The kit is supplied with four vials - a vial containing 3.2 mg of conjugated antibody in 2 ml saline, a vial containing 2 ml 50mM sodium acetate, a vial containing phosphate buffer, and a fourth empty reaction vial. Prior to labeling, a volume of sodium acetate buffer equivalent to 1.2 times the volume of the tracer solution is transferred to the reaction vial. Then 5.5 mCi (203.5 MBq) indium-111 or 40mCi (1.48 GBq) yttrium-90 is added to the reaction vial and mixed thoroughly without shaking. Next, 1.3 ml of conjugated antibody is added. The mixture is incubated for exactly 30min for indium-111 and for 5 min with yttrium-90 labeling, followed by the addition of enough phosphate buffer to make the final volume 10 ml. The labeling yield is determined by ITLC-SG with 0.9% saline as the mobile phase. Labeling efficiency should be greater than 95%.[4]
Administration
In order to qualify for ibritumomab, a patient needs to have bone marrow involvement of < 25% and > 15% bone marrow cellularity. Since ibritumomab is known to cause cytopenia, platelet and neutrophil counts are also taken pretreatment. Refractory/relapsed patients should have platelet counts of 100,000 per cubic millimetre (100,000/cmm) or greater; consolidation patients should have counts of 150,000/cmm or greater. Since a murine antibody is used, the patient might also be tested for human anti mouse antibodies (HAMA). Having bulky disease does not disqualify a patient.
The ibritumomab regimen takes 7–9 days. An imaging dose of the drug is no longer required in the U.S. Rituxan 250 mg/sq.m is given day 1, then on day 7-9 the Rituxan dose is repeated and Zevalin given within four hours. The dose of Zevalin 0.4 mCi/kg (= 14.8MBq/kg) if platelet counts are above 150,000/cmm; 0.3 mCi/kg (= 11.1MBq/kg) if 100,000-150,000/cmm. The Zevalin dose never exceeds 32 mCi (= 1184MBq).[5]
Ibritumomab tiuxetan is administered by intravenous infusion which usually lasts around 10 minutes. Only acrylic shielding is needed, not lead. A trained radiographer performs the infusion and safely disposes of waste.
Efficacy
Treatment with ibritumomab showed higher response rates in clinical trials compared to treatment with only rituximab (similar to ibritumomab, but without the attached radioisotope), and showed very promising results for patients who no longer respond to rituximab.
In patients with relapsed or refractory low-grade, follicular, or transformed B-cell NHL, where no prior anti-CD20 therapy was allowed, the ORR was 83% / 55% and CR was 38% / 18%, comparing ibritumomab to rituximab.
Recently, extended follow-up data for the ZEVALIN ([90Y]-ibritumomab tiuxetan) First-line Indolent (FIT) study presented at the American Society of Hematology (ASH) Annual Meeting demonstrated the continued improvement in progression-free survival (PFS) following ibritumomab consolidation therapy for patients with follicular B-cell non-Hodgkin's lymphoma who achieved a response to first-line therapy over chemotherapy alone. Additionally, ibritumomab consolidation did not adversely affect the use of various effective second-line treatments including stem cell transplants in patients who relapsed.
In a Phase II study on patients with relapsed and refractory mantle cell lymphoma, the OR was 42% and CR was 26%.
A study demonstrated that rituximab followed by single agent ibritumomab in a front-line setting for patients with MALT lymphoma and low-grade follicular lymphoma that primarily involved the conjunctiva or orbit, produced a complete response rate of 83 percent.
History
Developed by the IDEC Pharmaceuticals, now part of Biogen Idec, ibritumomab tiuxetan was the first radioimmunotherapy drug approved by the Food and Drug Administration (FDA) in 2002 to treat cancer. It was approved for the treatment of patients with relapsed or refractory, low‑grade or follicular B‑cell non‑Hodgkin's lymphoma (NHL), including patients with rituximab refractory follicular NHL.[6]
In September 2009, ibritumomab received approval from the FDA for an expanded label for the treatment of patients with previously untreated follicular non-Hodgkin's Lymphoma (NHL), who achieve a partial or complete response to first-line chemotherapy.[7]
Costs
Ibritumomab is currently under patent protection and not available in generic form. It is currently the most expensive drug available given in a single dose, costing over US$ 37,000 (€ 30,000) for the average dose. However, ibritumomab is essentially an entire course of lymphoma therapy which is delivered in 7–9 days, with one visit for pre-dosing Rituxan, and one visit a week later for the actual Zevalin therapeutic dose preceded by Rituxan. Compared to other monoclonal antibody treatments (many of which are well over US$ 40,000 for a course of therapy), this drug is priced in the middle for many of these therapies.
See also
- Tositumomab, an alternative radioimmunotherapy treatment for non-Hodgkin's lymphoma.
External links
- http://www.zevalin.com/ - Official Zevalin web site
- http://www.spectrumpharm.com/ - Spectrum Pharmaceuticals, Inc. web site
References
- ↑ Ibritumomab: Pronunciation
- ↑ Milenic, Diane E.; Brady, Erik D.; Brechbiel, Martin W. (June 2004). "Antibody-targeted radiation cancer therapy". Nat Rev Drug Discov. 3 (6): 488–499. doi:10.1038/nrd1413. ISSN 1474-1776. PMID 15173838.
- ↑ WHO Drug Information
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/ibriide021902LB.pdf
- ↑ Ibritumomab: Indications
- ↑ Grillo-López, Antonio J (2002). "Zevalin: the first radioimmunotherapy approved for the treatment of lymphoma". Expert Review of Anticancer Therapy. 2 (5): 485–493. doi:10.1586/14737140.2.5.485. ISSN 1473-7140.
- ↑ Schaefer, N. G.; Huang, P.; Buchanan, J. W.; Wahl, R. L. (2011). "Radioimmunotherapy in Non-Hodgkin Lymphoma: Opinions of Nuclear Medicine Physicians and Radiation Oncologists". Journal of Nuclear Medicine. 52 (5): 830–838. doi:10.2967/jnumed.110.085589. ISSN 0161-5505.