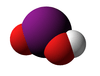
Iodous acid
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
iodous acid | |||
| Identifiers | |||
| 12134-99-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 145806 | ||
| ECHA InfoCard | 100.032.011 | ||
| PubChem | 166623 | ||
| |||
| |||
| Properties | |||
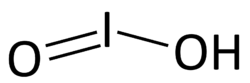
| HIO2 | |||
| Molar mass | 159.911 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Iodous acid is the chemical compound with the formula HIO2. Its salts are named iodites; these are exceedingly unstable and have been observed but never isolated.[1]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
This article is issued from Wikipedia - version of the 8/19/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.