Kynurenine 3-monooxygenase
| kynurenine 3-monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of the kynurenine 3-monooxygenase dimer, generated from 4J34.[1] One monomer is depicted in cartoon format (cyan) and the second monomer is displayed in ribbon format (green). The flexible linker regions (residues 96-104) are colored red. Flavin adenine dinucleotide (FAD) is shown as spheres color-coded according to atom type. | |||||||||
| Identifiers | |||||||||
| EC number | 1.14.13.9 | ||||||||
| CAS number | 9029-61-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a kynurenine 3-monooxygenase (EC 1.14.13.9) is an enzyme that catalyzes the chemical reaction
Kynurenine 3-monooxygenase is the expression product of the KMO (gene). The systematic name of this enzyme class is L-kynurenine, NADPH:oxygen oxidoreductase (3-hydroxylating). Other names in common use include kynurenine 3-hydroxylase, kynurenine hydroxylase, and L-kynurenine-3-hydroxylase. It participates in tryptophan metabolism through the kynurenine catabolic pathway. This enzyme belongs to the family of oxidoreductases, to be specific, those acting on paired donors, with O2 as the oxidant. Kynurenine 3-monooxygenase catalyzes the insertion of molecular oxygen into the aromatic ring of kynurenine to produce 3-hydroxy-L-kynurenine.[2] It employs one cofactor, FAD. Kynurenine 3-monooxygenase serves as an important branch point in the kynurenine pathway and, as a result, is an attractive drug target for immunological, neurodegenerative, and neuroinflammatory diseases.[3] Currently, most research on the kynurenine 3-monooxygenase enzyme has been focused primarily on rat models[4] and in yeast,[5] both of which have been demonstrated to have high sequence homology with the human kynurenine 3-monooxygenase protein. Studies have shown the beneficial effects of enzyme inhibition in these eukaryotic kynurenine 3-monooxygenase active sites, thus making this enzyme an attractive target for human drug design.[3][5]
Structure
Kynurenine 3-monooxygenase is a dimer containing asymmetric subunits[5] and has one FAD-binding domain as its prosthetic group.[3] Kynurenine 3-monooxygenase contains a linker region involved in substrate binding following a second strand of an antiparallel β-sheet, a six-stranded antiparallel β-sheet domain, and an α-helix at the carboxy-terminal.[5] The hydrophobic C-terminus acts as the mitochondrial anchoring domain and participates in enzymatic activity.[6]
Active Site
While no scientific literature reports a crystal image of a kynurenine 3-monooxygenase complex with L-kynurenine, structural studies of the enzyme in yeast co-crystallized with UPF 648 reveal how the FAD cofactor and substrate are bound in the active site.[1] Chemical similarities between UPF 648 and L-kynurenine suggest that the substrate binds adjacent to the re-face of the flavoprotein. A loop containing the residues Pro 321 – Gln 325 is believed to be the oxygen-binding site above the re-side of the FAD prosthetic group.[5]
Each monomer contains a conserved hydrophobic pocket (residues Leu 221, Met 230, Ile 232, Leu 234, Phe 246, Pro 321, Phe 322) positioned around the substrate’s aromatic benzene moiety.[5] A conserved Gln 325 polar residue is also involved in hydrogen bonding on the L-kynurenine carbonyl group, as well as on the hydrogen on the FAD N3 atom.[1] Arg 83 and Tyr 97 also form polar contacts with the carboxylate in the amino acid moiety on the substrate.[7]
Mechanism
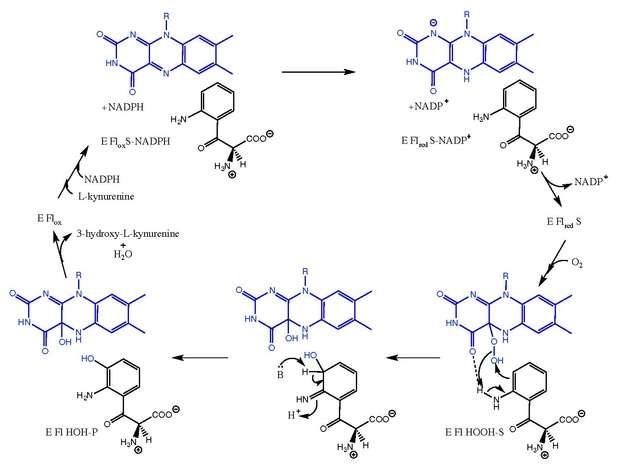
Kynurenine-3-monooxygenase catalyzes the hydroxylation of L-kynurenine to 3-hydroxy-L-kynurenine with concomitant interconversion of NADPH to NADP+. The reaction mechanism is not entirely known, but is believed to follow mechanisms related to the flavin-dependent monooxygenases.[8] After L-Kynurenine binds, NADPH reduces FAD and leaves as NADP+. Oxygen then binds and creates an L-Kynurenine-FAD-hydroperoxide intermediate.[5][9] This intermediate is the electrophilic source for the hydroxylation reaction, yielding a primary ketimine form of the product and the C4a-hydroxy-FAD.[10] Tautomerization yields 3-hydroxy-L-kynurenine in complex with the enzyme (E Fl HOH-P). Dissociation of 3-hydroxy-L-kynurenine and H2O leads to the free enzyme (E Flox).
Biological Function
Kynurenine 3-monooxygenase catalyzes the conversion of L-kynurenine to 3-hydroxy-L-kynurenine, an important bioactive metabolite in the kynurenine pathway. The kynurenine pathway is responsible for >95% of tryptophan oxidative degradation.[11] L-Kynurenine is an important branch point of this metabolic pathway, being converted into the neurotoxin 3-hydroxy-L-kynurenine via kynurenine 3-monooxygenase, the neuroprotectant kynurenic acid through kynurenine amino transferases, or anthranilic acid by kynureninase.[12]
Kynurenine 3-monooxygenase regulates the downstream production of quinolinic acid, which can generate reactive free radicals[13] and activates the NMDA subtype of glutamate receptors, producing excitotoxic lesions in the central nervous system of mammals.[14][15] Quinolinic acid is also the bioprecursor of NAD+.[12]
Inhibition of kynurenine 3-monooxygenase leads to an increase of kynurenic acid in the kynurenine pathway. This metabolite functions as an antagonist of the α7 nicotinic acetylcholine receptor and as an agonist at the glycine site of the NMDA receptor.[16] As a result, regulation at the kynurenine 3-monooxygenase enzyme determines the neurotoxic and neuroprotective potential of the kynurenine pathway.
Disease Relevance
Kynurenine 3-monooxygenase is an attractive drug target for several neurodegenerative and neuroinflammatory diseases, especially Huntington's, Alzheimer's, and Parkinson's disease. Administration of potent enzyme inhibitors has demonstrated promising pharmacological results.[3][5] Specifically, genetic elimination of the kynurenine 3-monooxygenase enzyme has been shown to suppress toxicity of the huntingtin protein in yeast[17] and Drosophila[18] models of Huntington's disease.
Kynurenine 3-monooxygenase deficiency, which can be caused by genetic polymorphisms, cytokines, or both,[19] leads to an accumulation of kynurenine and to a shift within the tryptophan metabolic pathway towards kynurenic acid and anthranilic acid. Recent research suggests that hyperphysiologic concentrations of kynurenine in kynurenine 3-monooxygenase-deficient patients results in a shift towards kynurenic acid production, believed to be related to cognitive deficits in predictive pursuit and visuospatial working memory.[20] Kynurenine-3-monooxygenase deficiency is associated with disorders of the brain (e.g. schizophrenia, tic disorders) and of the liver.[21][22][23][24][25]
References
- 1 2 3 Amaral, M. (2014). "Crystal Structure of kynurenine 3-monooxygenase - truncated at position 394 plus HIS tag cleaved". doi:10.2210/pdb4j34/pdb.
- ↑ Filippini, Graziella Allegri; Costa, Carlo V. L.; Bertazzo, Antonella; International Meeting on Tryptophan Research (1998). "Recent advances in tryptophan research : tryptophan and serotonin pathways.". Advances in Experimental Medicine and Biology. 398. doi:10.1007/978-1-4613-0381-7.
- 1 2 3 4 Smith, Jason R.; Jamie, Joanne F.; Guillemin, Gilles J. (February 2016). "Kynurenine-3-monooxygenase: a review of structure, mechanism, and inhibitors". Drug Discovery Today. 21 (2): 315–324. doi:10.1016/j.drudis.2015.11.001. ISSN 1359-6446.
- ↑ Horn, U.; Ullrich, V.; Staudinger, H.J (1971). "Purification and characterization of L-kynurenine 3-hydroxylase (EC 1.14.1.2.) from rat liver". Hoppe-Seyler’s. Z. Physiol. Chem. 352 (6): 837–842. PMID 5087636.
- 1 2 3 4 5 6 7 8 9 Amaral, M; Levy, C; Heyes, DJ; Lafite, P; Outeiro, TF; Giorgini, F; Leys, D; Scrutton, NS (2013). "Structural basis of kynurenine 3-monooxygenase inhibition". Nature. 496: 382–385. doi:10.1038/nature12039.
- ↑ Hirai, KH, et al. (2010). "Dual role of the carboxyl-terminal region of pig liver L-kynurenine 3-monooxygenase: mitochondrial-targeting signal and enzymatic activity". J. Biochem. 148 (6): 639–650. doi:10.1093/jb/mvq099.
- ↑ Mole D, et al. (2016). "Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis". Nature Medicine. 22: 202–209. doi:10.1038/nm.4020.
- ↑ Breton, J.; et al. (2000). "Functional characterization and mechanism of action of recombinant human kynurenine 3-hydroxylase". Eur. J. Biochem. 267: 1092–1099. doi:10.1046/j.1432-1327.2000.01104.x. PMID 10672018.
- 1 2 Crozier-Reabe, KR; et al. (2008). "Kynurenine 3-monooxygenase from Pseudomonas fluorescens: substrate-like inhibitors both stimulate flavin reduction and stabilize the flavin-peroxo intermediate yet result in the production of hydrogen peroxide". Biochemistry. 47: 12420–12433. doi:10.1021/bi8010434. PMID 18954092.
- 1 2 Entsch, B, et al. (1976). "Flavin-oxygen derivatives involved in the hydroxylation of p-hydroxybenzoate hydroxylase". J. Biol. Chem. 251: 2550–2563. PMID 816794.
- ↑ Thevandavakkam, M.A; et al. (2010). "Targeting kynurenine 3-monooxygenase (KMO). Implications for therapy in Huntington's disease". CNS Neurol. Disord. Drug Targets. 9: 791–800. doi:10.2174/187152710793237430. PMID 20942784.
- 1 2 Giorgini F, Huang SY, Sathyasaikumar KV, et al. (2013). "Targeted Deletion of Kynurenine 3-Monooxygenase in Mice: A NEW TOOL FOR STUDYING KYNURENINE PATHWAY METABOLISM IN PERIPHERY AND BRAIN". The Journal of Biological Chemistry. 288 (51): 36554–36566. doi:10.1074/jbc.M113.503813.
- ↑ Rios, C.; Santamaria, A. (1991). "Quinolinic acid is a potent lipid peroxidant in rat brain homogenates". Neurochem. Res. 16: 1139–1143. doi:10.1007/bf00966592. PMID 1686636.
- ↑ Stone, T. W.; Perkins, M. N. (1981). "Quinolinic acid: A potent endogenous excitant at amino acid receptors in CNS". Eur. J. Pharmacol. 72: 411–412. doi:10.1016/0014-2999(81)90587-2. PMID 6268428.
- ↑ Schwarcz, R.; Bruno, J. P.; Muchowski, P. J.; Wu, H. Q. (2012). "Kynurenines in the mammalian brain. When physiology meets pathology". Nat. Rev. Neurosci. 13: 465–477. doi:10.1038/nrn3257. PMID 22678511.
- ↑ Hilmas, C.; Pereira, E. F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E. X. (2001). "The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression:Physiopathological implications". J. Neurosci. 21: 7463–7473. PMID 11567036.
- ↑ Giorgini, F.; Guidetti, P.; Nguyen, Q.; Bennett, S. C.; Muchowski, P. J. (2005). "A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease". Nat. Genet. 37: 526–531. doi:10.1038/ng1542. PMC 1449881
 . PMID 15806102.
. PMID 15806102. - ↑ Campesan, S.; Green, E. W.; Breda, C.; Sathyasaikumar, K. V.; Muchowski, P. J.; Schwarcz, R.; Kyriacou, C. P.; Giorgini, F (2011). "The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease". Curr. Biol. 21: 961–966. doi:10.1016/j.cub.2011.04.028. PMC 3929356
 . PMID 21636279.
. PMID 21636279. - ↑ Müller, N; Myint, AM; Schwarz, MJ (2010). "Inflammatory Biomarkers and Depression". Neurotox Res. 19 (2): 308–318. doi:10.1007/s12640-010-9210-2. PMID 20658274.
- ↑ Wonodi I, Colin-Stine O, Sathyasaikumar KV, et al. (2011). "Downregulated Kynurenine 3-Monooxygenase Gene Expression and Enzyme Activity in Schizophrenia and Genetic Association With Schizophrenia Endophenotypes". Arch Gen Psychiatry. 68 (7): 665–674. doi:10.1001/archgenpsychiatry.2011.71. PMC 3855543
 . PMID 21727251.
. PMID 21727251. - ↑ Holtze M, Saetre P, Engberg G, et al. (2012). "Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls". J Psychiatry Neurosci. 37 (1): 53–57. doi:10.1503/jpn.100175. PMC 3244499
 . PMID 21693093.
. PMID 21693093. - ↑ Campbell, Brian M.; Charych, Erik; Lee, Anna W.; Möller, Thomas (2014). "Kynurenines in CNS disease: regulation byinflammatory cytokines". Frontiers in Neuroscience. Neuroendocrine Science. 8 (12). doi:10.3389/fnins.2014.00012.
- ↑ Hoekstra, PJ; Anderson, GM; Troost, PW (2007). "Plasma kynurenine and related measures in tic disorder patients". Eur Child Adolesc Psychiatry. 16: Suppl 1:71–7. doi:10.1007/s00787-007-1009-1. PMID 17665285.
- ↑ Buness A, Roth A, Herrmann A, Schmitz O, Kamp H, et al. (2014). "Identification of Metabolites, Clinical Chemistry Markers and Transcripts Associated with Hepatotoxicity". PLoS ONE. 9 (5): e97249. doi:10.1371/journal.pone.0097249.
- ↑ Yukiko, Hirata; Takashi, Kawachi; Takashi, Sugimura (2 October 1967). "Fatty liver induced by injection of L-tryptophan". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 144 (2): 233–241. doi:10.1016/0005-2760(67)90153-1.
- Foucher AL, McIntosh A, Douce G, Wastling J, Tait A, Turner CM (2006). "A proteomic analysis of arsenical drug resistance in Trypanosoma brucei". Proteomics. 6 (9): 2726–32. doi:10.1002/pmic.200500419. PMID 16526094.
- Okamoto H, Hayaishi O (1967). "Flavin adenine dinucleotide requirement for kynurenine hydroxylase of rat liver mitochondria". Biochem. Biophys. Res. Commun. 29 (3): 394–9. doi:10.1016/0006-291X(67)90469-X. PMID 6076241.
- SAITO Y, HAYAISHI O, ROTHBERG S (1957). "Studies on oxygenases; enzymatic formation of 3-hydroxy-L-kynurenine from L-kynurenine". J. Biol. Chem. 229 (2): 921–34. PMID 13502353.