Lead zirconate titanate
 | |
| Names | |
|---|---|
| IUPAC name
Lead zirconium titanate | |
| Other names
PZT | |
| Identifiers | |
| 12626-81-2 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.032.467 |
| EC Number | 235-727-4 |
| PubChem | 159452 |
| |
| Properties | |
| Pb[ZrxTi1-x]O3 (0≤x≤1) | |
| Molar mass | 303.065 to 346.4222 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lead zirconate titanate is an intermetallic inorganic compound with the chemical formula Pb[ZrxTi1-x]O3 (0≤x≤1). Also called PZT, it is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. PZT is a white solid that is insoluble in all solvents.
Electroceramic properties
Being piezoelectric, PZT develops a voltage (or potential difference) across two of its faces when compressed (useful for sensor applications), or physically changes shape when an external electric field is applied (useful for actuator applications). The dielectric constant of PZT can range from 300 to 3850, depending upon orientation and doping.[1]
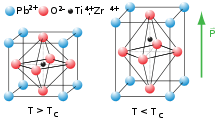
Being pyroelectric, this material develops a voltage difference across two of its faces under changing temperature conditions; consequently, PZT can be used as a heat sensor. PZT is also ferroelectric, which means it has a spontaneous electric polarization (electric dipole) that can be reversed in the presence of an electric field.
The material features an extremely large dielectric constant at the morphotropic phase boundary (MPB) near x = 0.52.[2]
Some formulations are ohmic until at least 250 kV/cm (25 MV/m), after which current grows exponentially with field strength before reaching avalanche breakdown; but PZT exhibits time-dependent dielectric breakdown — breakdown may occur under constant-voltage stress after minutes to hours, depending on voltage and temperature, so its dielectric strength depends on the time scale over which it is measured.[3] Other formulations have dielectric strengths measured in the 8–16 MV/m range.[4]
Uses
PZT-based materials are components of ultrasound transducers and ceramic capacitors, STM/AFM actuators (tubes).
PZT is used to make ultrasound transducers and other sensors and actuators, as well as high-value ceramic capacitors and FRAM chips. PZT is also used in the manufacture of ceramic resonators for reference timing in electronic circuitry. In 1975 Sandia National Laboratories created anti-flash goggles featuring PZLT to protect aircrew from burns and blindness in case of a nuclear explosion.[5] The PLZT lenses could turn opaque in less than 150 microseconds.
Commercially, it is usually not used in its pure form, rather it is doped with either acceptors, which create oxygen (anion) vacancies, or donors, which create metal (cation) vacancies and facilitate domain wall motion in the material. In general, acceptor doping creates hard PZT while donor doping creates soft PZT. Hard and soft PZT's generally differ in their piezoelectric constants. Piezoelectric constants are proportional to the polarization or to the electrical field generated per unit of mechanical stress, or alternatively is the mechanical strain produced by per unit of electric field applied. In general, soft PZT has a higher piezoelectric constant, but larger losses in the material due to internal friction. In hard PZT, domain wall motion is pinned by the impurities thereby lowering the losses in the material, but at the expense of a reduced piezoelectric constant.
Varieties of PZTs
One of the commonly studied chemical composition is PbZr0.52Ti0.48O3. The increased piezoelectric response and poling efficiency near to x = 0.52 is due to the increased number of allowable domain states at the MPB. At this boundary, the 6 possible domain states from the tetragonal phase <100> and the 8 possible domain states from the rhombohedral phase <111> are equally favorable energetically, thereby allowing a maximum 14 possible domain states.
Like structurally similar lead scandium tantalate and barium strontium titanate, PZT can be used for manufacture of uncooled staring array infrared imaging sensors for thermographic cameras. Both thin film (usually obtained by chemical vapor deposition) and bulk structures are used. The formula of the material used usually approaches Pb1.1(Zr0.3Ti0.7)O3 (called PZT 30/70). Its properties may be modified by doping it with lanthanum, resulting in lanthanum-doped lead zirconium titanate (PLZT, also called lead lanthanum zirconium titanate), with formula Pb0.83La0.17(Zr0.3Ti0.7)0.9575O3 (PLZT 17/30/70).[6]
See also
References
- ↑ An Overview of the Properties of Different Piezoceramic Materials
- ↑ Rouquette, J.; Haines, J.; Bornand, V.; Pintard, M.; Papet, Ph; Bousquet, C.; Konczewicz, L.; Gorelli, F. A.; Hull, S. (2004). "Pressure tuning of the morphotropic phase boundary in piezoelectric lead zirconate titanate". Physical Review B. 70 (1): 014108. doi:10.1103/PhysRevB.70.014108.
- ↑ Moazzami, Reza; Hu, Chenming; Shepherd, William H. (September 1992). "Electrical Characteristics of Ferroelectric PZT Thin Films for DRAM Applications" (PDF). IEEE Transactions on Electron Devices. 39 (9): 2044. doi:10.1109/16.155876.
- ↑ B. Andersen, E. Ringgaard, T. Bove, A. Albareda, and R. Pérez (2000). "Performance of Piezoelectric Ceramic Multilayer Components Based on Hard and Soft PZT" (PDF). Proceedings of Actuator 2000: 419–422.
- ↑ Cutchen, J. Thomas; Harris, Jr., James O.; Laguna, George R. (1975). "PLZT electrooptic shutters: applications". Applied Optics. 14 (8): 1866–1873. doi:10.1364/AO.14.001866.
- ↑ Liu, W.; Jiang, B.; Zhu, W. (2000). "Self-biased dielectric bolometer from epitaxially grown Pb(Zr,Ti)O3 and lanthanum-doped Pb(Zr,Ti)O3 multilayered thin films". Applied Physics Letters. 77 (7): 1047–1049. doi:10.1063/1.1289064.