Mangiferin
 | |
| Names | |
|---|---|
| IUPAC name
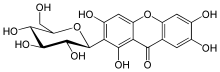
(1S)-1,5-Anhydro-1-(1,3,6,7-tetrahydroxy-9-oxo-9H-xanthen-2-yl)-D-glucitol | |
| Identifiers | |
| 4773-96-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:6682 |
| ChEMBL | ChEMBL455364 |
| ChemSpider | 4444966 |
| ECHA InfoCard | 100.153.319 |
| KEGG | C10077 |
| PubChem | 5281647 |
| UNII | 1M84LD0UMD |
| |
| |
| Properties | |
| C19H18O11 | |
| Molar mass | 422.34 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Mangiferin is a xanthonoid. This molecule is a glucoside of norathyriol.
Natural occurrences
It is found in mangoes,[1] in Iris unguicularis,[2] Anemarrhena asphodeloides rhizomes,[3] and in Bombax Ceida.[4] It is also found in the genera Salacia and Cyclopia.
Among the group of Asplenium hybrids known as the "Appalachian Asplenium complex", mangiferin and isomangiferin are produced only by Asplenium montanum and its hybrid descendants. The distinctive gold-orange fluorescence of these compounds under ultraviolet light has been used to aid in the chromatographic identification of hybrid Aspleniums.[5]
Research
Laboratory study has identified a variety of potential pharmacology associated with mangiferin including antimicrobial and antioxidant activities,[6] inhibitory effects on type II 5α-reductase in vitro,[7] and gastroprotective[8] and antidiabetic[3] effects in rodents.
See also
- Ethanolic extract of mango peel
References
- ↑ Barreto J.C.; Trevisan M.T.S.; Hull W.E.; Erben G.; De Brito E.S.; Pfundstein B.; Würtele G.; Spiegelhalder B.; Owen R.W. (2008). "Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.)". Journal of Agricultural and Food Chemistry. 56 (14): 5599–5610. doi:10.1021/jf800738r. PMID 18558692.
- ↑ Atta-Ur-Rahman, _; Hareem, Sumaira; Iqbal Choudhary, Muhammad; Sener, Bilge; Abbaskhan, Ahmed; Siddiqui, Hina; Anjum, Shazia; Orhan, Ilkay; Gurbuz, Ilhan; Ayanoglu, Filiz (2010). "New and Known Constituents from Iris unguicularis and Their Antoioxidant Activity". Heterocycles. 82: 813. doi:10.3987/COM-10-S(E)6.
- 1 2 Miura, T.; Ichiki, H.; Hashimoto, I.; Iwamoto, N.; Kato, M.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Okada, M.; Ishida, T.; Tanigawa, K. (2001). "Antidiabetic Activity of a Xanthone Compound, Mangiferin". Phytomedicine. 8 (2): 85–87. doi:10.1078/0944-7113-00009. PMID 11315760.
- ↑ Dar, A; Faizi, S; Naqvi, S; Roome, T; Zikr-Ur-Rehman, S; Ali, M; Firdous, S; Moin, S. T. (2005). "Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship". Biological & Pharmaceutical Bulletin. 28 (4): 596–600. doi:10.1248/bpb.28.596. PMID 15802793.
- ↑ Smith, Dale M.; Harborne, Jeffrey B. (1971). "Xanthones in the Appalachian Asplenium complex". Phytochemistry. 10 (9): 2117–2119. doi:10.1016/S0031-9422(00)97205-4.
- ↑ Stoilova, I.; Gargova, S.; Stoyanova, A.; Ho, L. (2005). "Antimicrobial and Antioxidant Activity of the Polyphenol Mangiferin". Herba Polonica. 51 (1/2): 37–44. ISSN 0018-0599.
- ↑ Wang, X.; Liao, J.; Yin, D.; Zhan, F.; Dai, S.; Xie, G.; Sang, X. (2010). "Establishment of a Novel Model for Studying the Effects of Extracts of Chinese Herb Medicine on Human Type II 5-alpha-Reductase in Vitro". Yakugaku Zasshi. 130 (9): 1207–1214. doi:10.1248/yakushi.130.1207. PMID 20823678.
- ↑ Carvalho, A.; Guedes, M.; De Souza, A.; Trevisan, M.; Lima, A.; Santos, F. V.; Rao, V. (2007). "Gastroprotective Effect of Mangiferin, a Xanthonoid from Mangifera indica, against Gastric Injury Induced by Ethanol and Indomethacin in Rodents". Planta Medica. 73 (13): 1372–1376. doi:10.1055/s-2007-990231. PMID 17918041.