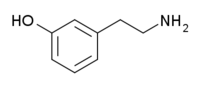
meta-Tyramine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-(2-Aminoethyl)phenol | |
| Other names
m-Tyramine; 3-Tyramine; 3-Hydroxyphenylethylamine | |
| Identifiers | |
| 588-05-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 11008 |
| ECHA InfoCard | 100.197.155 |
| PubChem | 11492 |
| |
| |
| Properties | |
| C8H11NO | |
| Molar mass | 137.18 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
meta-Tyramine, also known as m-tyramine or 3-tyramine as well as 3-hydroxyphenylethylamine, is an endogenous monoamine compound and trace amine of the phenethylamine class.[1][2][3] It is a positional isomer of para-tyramine, and similarly to it, has effects on the adrenergic and dopaminergic systems.[4][5]
See also
References
- ↑ Boulton AA, Huebert ND (November 1981). "Biosynthesis of some urinary trace amines in the rat and the human". Research Communications in Chemical Pathology and Pharmacology. 34 (2): 295–310. PMID 7335956.
- ↑ Dyck LE, Juorio AV, Boulton AA (June 1982). "The in vitro release of endogenous m-tyramine, p-tyramine and dopamine from rat striatum". Neurochemical Research. 7 (6): 705–16. doi:10.1007/bf00965523. PMID 7121718.
- ↑ Sardar A, Juorio AV, Boulton AA (June 1987). "The concentration of p- and m-tyramine in the rat mesolimbic system: its regional distribution and effect of monoamine oxidase inhibition". Brain Research. 412 (2): 370–4. doi:10.1016/0006-8993(87)91145-0. PMID 3607473.
- ↑ Dyck LE, Kazakoff CW, Dourish CT (October 1982). "The role of catecholamines, 5-hydroxytryptamine and m-tyramine in the behavioural effects of m-tyrosine in the rat". European Journal of Pharmacology. 84 (3-4): 139–49. doi:10.1016/0014-2999(82)90196-0. PMID 7173317.
- ↑ McQuade PS, Wood PL (1984). "The effects of administration of meta-tyramine and para-tyramine on dopamine and its metabolites in the rat striatum". Progress in Neuro-psychopharmacology & Biological Psychiatry. 8 (4-6): 705–9. doi:10.1016/0278-5846(84)90042-3. PMID 6531442.
This article is issued from Wikipedia - version of the 6/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.