Metal–air electrochemical cell
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous electrolyte.[1][2]
Types
The Li–air battery discharge reaction between Li and oxygen Li2O, according to 4Li + O2 → 2Li2O, has an open-circuit voltage of 2.91 V and a theoretical specific energy of 5,210 watt-hours per kilogram (Wh/kg). Since oxygen is not stored in the battery, the theoretical specific energy excluding oxygen is 11,140 Wh/kg (40.1 MJ/kg). Compare this to the figure of 12,200 Wh/kg (44 MJ/kg) for gasoline (see Petrol energy content).
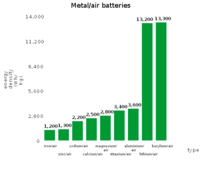
| Metal–air battery | Theoretical specific energy, Wh/kg (including oxygen) |
Theoretical specific energy, Wh/kg (excluding oxygen) |
Calculated open-circuit voltage, V |
|---|---|---|---|
| Aluminium–air | 4300[3] | 8140[4] | 1.2 |
| Germanium–air | 1480 | 7850 | 1 |
| Calcium-air | 2990 | 4180 | 3.12 |
| Iron–air | 1431 | 2044 | 1.3 |
| Lithium–air | 5210 | 11140 | 2.91 |
| Magnesium air | 2789 | 6462 | 2.93 |
| Potassium-air | 935[5][6] | 1700[Note 1] | 2.48[5][6] |
| Sodium–air | 1677 | 2260 | 2.3[7][8] |
| Silicon–air | 4217 | 9036 | 1.6 |
| Tin–air at 1000 K [9] | 860 | 6250 | 0.95 |
| Zinc-air | 1090 | 1350 | 1.65 |
Iron-air
Iron-air rechargeable batteries are an attractive technology with the potential of grid-scale energy storage. The main raw-material of this technology is iron oxide (rust) which is abundant, non-toxic, inexpensive and environmentally friendly.[10] Most of the batteries being developed right now utilize iron oxide (mostly powders) to generate/store hydrogen via the Fe/FeO reduction/oxidation (redox) reaction (Fe + H2O = FeO + H2).[11] In conjunction with a fuel cell this enables the system to behave as a rechargeable battery creating H2O/H2 via production/consumption of electricity.[12] Furthermore, this technology has minimal environmental impact as it could be used to store energy from intermittent solar and wind power sources, developing an energy system with low carbon dioxide emissions.
The way the system works can start by using the Fe/FeO redox reaction, then the hydrogen created during the oxidation of iron can be consumed by a fuel cell in conjunction with oxygen from the air to create electricity. When electricity must be stored, hydrogen generated from water by operating the fuel cell in reverse is consumed during the reduction of the iron oxide to metallic iron.[11][12] The combination of both of this cycles is what makes the system operate as an iron-air rechargeable battery.
Limitations of this technology come from the materials used. Generally, iron oxide powder beds are selected, however, rapid sintering and pulverization of the powders limit the ability to achieve a high number of cycles resulting in a lower capacity. Other methods, such as 3D-Printing[13] and freeze casting,[14][15] currently under investigation seek to enable the creation of architecture materials to allow for high surface area and volume changes during the redox reaction.
See also
References
- ↑ Metal Air Batteries, Half a Fuel Cell?
- ↑ "METAL-AIR BATTERIES Lithium, Aluminum, Zinc, and Carbon" (PDF). Retrieved 2013-04-04.
- ↑ "Electrically Rechargeable Metal-Air Batteries (ERMAB)". Retrieved 25 March 2012.
- ↑ "Batteries for Oxygen Concentrators".
- 1 2 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- 1 2 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- ↑ "Electrochemical properties of room temperature sodium–air batteries with non-aqueous electrolyte".
- ↑ "BASF investigating sodium-air batteries as alternative to Li-air; patent application filed with USPTO".
- ↑ HyungKuk Ju and Jaeyoung Lee*, High-temperature liquid Sn-air energy storage cell, Journal of Energy Chemistry, 24 (2015) 614.
- ↑ Narayanan, S. R.; Prakash, G. K. Surya; Manohar, A.; Yang, Bo; Malkhandi, S.; Kindler, Andrew (2012-05-28). "Materials challenges and technical approaches for realizing inexpensive and robust iron–air batteries for large-scale energy storage". Solid State Ionics. "Fuel Cells-Energy Conversion" Proceedings of Symposium X EMRS Spring Meeting 2011E-MRS / MRS BILATERAL CONFERENCE on ENERGY,"Held at the E-MRS 2011 SPRING MEETING IUMRS ICAM 2011. 216: 105–109. doi:10.1016/j.ssi.2011.12.002.
- 1 2 Requies, J.; Güemez, M. B.; Gil, S. Perez; Barrio, V. L.; Cambra, J. F.; Izquierdo, U.; Arias, P. L. (2013-04-19). "Natural and synthetic iron oxides for hydrogen storage and purification". Journal of Materials Science. 48 (14): 4813–4822. doi:10.1007/s10853-013-7377-7. ISSN 0022-2461.
- 1 2 Ju, Young-Wan; Ida, Shintaro; Inagaki, Toru; Ishihara, Tatsumi (2011-08-01). "Reoxidation behavior of Ni–Fe bimetallic anode substrate in solid oxide fuel cells using a thin LaGaO3 based film electrolyte". Journal of Power Sources. 196 (15): 6062–6069. doi:10.1016/j.jpowsour.2011.03.086.
- ↑ Jakus, Adam E.; Taylor, Shannon L.; Geisendorfer, Nicholas R.; Dunand, David C.; Shah, Ramille N. (2015-12-01). "Metallic Architectures from 3D-Printed Powder-Based Liquid Inks". Advanced Functional Materials. 25 (45): 6985–6995. doi:10.1002/adfm.201503921. ISSN 1616-3028.
- ↑ Sepúlveda, Ranier; Plunk, Amelia A.; Dunand, David C. (2015-03-01). "Microstructure of Fe2O3 scaffolds created by freeze-casting and sintering". Materials Letters. 142: 56–59. doi:10.1016/j.matlet.2014.11.155.
- ↑ Durán, P.; Lachén, J.; Plou, J.; Sepúlveda, R.; Herguido, J.; Peña, J. A. (2016-11-16). "Behaviour of freeze-casting iron oxide for purifying hydrogen streams by steam-iron process". International Journal of Hydrogen Energy. The 5th Iberian Symposium on Hydrogen, Fuel Cells and Advanced Batteries (HYCELTEC 2015), 5–8 July 2015, Tenerife, Spain. 41 (43): 19518–19524. doi:10.1016/j.ijhydene.2016.06.062.
Notes
- ↑ Calculated from the specific energy density (including oxygen) value and 39.1 and 16 atomic weight data for K and O respectively for KO2
