Tetrahydromethanopterin
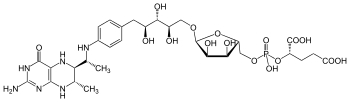 | |
| Identifiers | |
|---|---|
| 92481-94-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4573696 |
| PubChem | 5462234 |
| |
| |
| Properties | |
| C 30H 45N 6O 16P | |
| Molar mass | 776.682661 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetrahydromethanopterin (THMPT, H
4MPT) is a coenzyme in methanogenesis. It is the carrier of the C1 group as it is reduced to the methyl level, before transferring to the coenzyme M.[1]
Tetrahydrosarcinapterin (THSPT, H
4SPT) is a modified form of THMPT, wherein a glutamyl group linked to the 2-hydroxyglutaric acid terminus.
THMPT is the main platform for C1 transformations
N-Formylmethanofuran donates the C1 group to the N5 site of the pterin to give the formyl- THMPT.[2] The formyl group subsequently condenses intramolecularly to give methenyl- THMPT+
, which is then reduced to methylene- THMPT.[3] Methylene- MPT is subsequently converted, using coenzyme F420 as the electron source, to methyl- THMPT, catalyzed by F420-dependent methylene- THMPT reductase. Methyl- THMPT is the methyl donor to coenzyme M, a conversion mediated by methyl- THMPT:coenzyme M methyl-transferase.[1]
Comparison with tetrahydrofolic acid
THMPT is related to the better known tetrahydrofolic acid (THFA, H
4FA). The most important difference between THMPT and THFA is that THFA has an electron-withdrawing carbonyl group on the phenyl ring. As a consequence, methenyl- THMPT is more difficult to reduce than methenyl- THFA. Reduction is effected by a so-called iron-sulfur cluster free hydrogenase.[3] The cumbersome name distinguishes this hydrogenase from the so-called Fe-only hydrogenases that do contain Fe-S cluster.
References
- 1 2 Thauer RK (September 1998). "Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture". Microbiology. 144 (Pt 9): 2377–406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- ↑ Acharya P, Warkentin E, Ermler U, Thauer RK, Shima S (March 2006). "The structure of formylmethanofuran: tetrahydromethanopterin formyltransferase in complex with its coenzymes". J. Mol. Biol. 357 (3): 870–9. doi:10.1016/j.jmb.2006.01.015. PMID 16466742.
- 1 2 Korbas M, Vogt S, Meyer-Klaucke W, et al. (October 2006). "The iron-sulfur cluster-free hydrogenase (Hmd) is a metalloenzyme with a novel iron binding motif". J. Biol. Chem. 281 (41): 30804–13. doi:10.1074/jbc.M605306200. PMID 16887798.