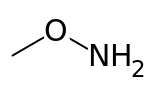
Methoxyamine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methoxyamine | |
| Other names
Methoxylamine; (Aminooxy)methane; O-Methylhydroxylamine | |
| Identifiers | |
| 593-56-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 3970 |
| PubChem | 4113 |
| |
| |
| Properties | |
| CH5NO | |
| Molar mass | 47.06 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Ammoniacal |
| Density | 1.0003 g/mL |
| Melting point | 42 °C (108 °F; 315 K) |
| Boiling point | 50 °C (122 °F; 323 K) |
| Miscible | |
| Vapor pressure | 297.5 mmHg at 25°C |
| Refractive index (nD) |
1.4164 |
| Hazards | |
| Safety data sheet | Santa Cruz (HCl) |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methoxyamine is the organic compound with the formula CH3ONH2. Also called O-methylhydroxylamine, it a colourless volatile liquid that is soluble in polar organic solvents and in water. It is a derivative of hydroxylamine with the hydroxyl hydrogen replaced by a methyl group. Alternatively, it can be viewed as a derivative of methanol with the hydroxyl hydrogen replaced by an amino group. It is an isomer of N-methylhydroxylamine and aminomethanol. It decomposes in an exothermic reaction (-56 kJ/mol) to methane and azanone unless stored as a hydrochloride salt.
Synthesis
Methoxyamine is prepared via O-alkylation of hydroxylamine derivatives. For example, it is obtained by O-methylation of acetone oxime followed by hydrolysis or the O-methylated oxime:[1]
- (CH3)2CNOCH3 + H2O → (CH3)2CO + H2NOCH3
The other broad method involves methanolysis of hydroxylamine sulfonates:
- H2NOSO3− + CH3OH → H2NOCH3 + HSO4−
Reactions
Like hydroxylamine, methoxyamine forms oximes upon treatment with ketones and aldehydes.
Methoxyamine is used as a synthon for NH2+. It undergoes deprotonation by methyl lithium to give CH3ONHLi. This N-lithio derivative is attacked by organolithium compounds to give, after hydrolysis, amines:[2]
- H2NOCH3 + CH3Li → LiHNOCH3 + CH4
- LiHNOCH3 + RLi → RNHLi + LiOCH3
- RNHLi + H2O → RNH2 + LiOH
Uses
Methoxyamine is an orally bioavailable small molecule inhibitor with potential adjuvant activity.[3] Methoxyamine covalently binds to apurinic/apyrimidinic (AP) DNA damage sites and inhibits base excision repair (BER), which may result in an increase in DNA strand breaks and apoptosis.[3] This agent may potentiate the anti-tumor activity of alkylating agents.
Examples of drugs incorporating the methoxyamine unit are brasofensine and gemifloxacin.
References
- ↑ Review: Houben-Weyl, Methoden der organische Chemie, vol 10.1, p 1186. Patent: Klein, Ulrich; Buschmann, Ernst; Keil, Michael; Goetz, Norbert; Hartmann, Horst "Process for preparing O-substituted hydroxylammonium salts." Ger. Offen. to BASF, (1994), DE 4233333 A1 19940407.
- ↑ Bruce J. Kokko, Scott D. Edmondson "O-Methylhydroxylamine" in eEROS, 2008. doi:10.1002/047084289X.rm192m.pub2
- 1 2 NCI
