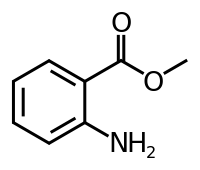
Methyl anthranilate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl 2-aminobenzoate | |
| Identifiers | |
| 134-20-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:73244 |
| ChemSpider | 13858096 |
| ECHA InfoCard | 100.004.667 |
| EC Number | 205-132-4 |
| KEGG | C20634 |
| |
| |
| Properties | |
| C8H9NO2 | |
| Molar mass | 151.165 |
| Density | 1.168 g/cm^3 |
| Melting point | 24 °C (75 °F; 297 K) |
| Boiling point | 256 °C (493 °F; 529 K) |
| Hazards | |
| Flash point | 104 °C (219 °F; 377 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methyl anthranilate, also known as MA, methyl 2-aminobenzoate, or carbomethoxyaniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2.
Chemical properties
It is a clear to pale yellow liquid with melting point 24 °C and boiling point 256 °C. It has a density of 1.168 g/cm3 at 20 °C.[1] It has a refractive index of 1.583 at 589 nm of wavelength and 20 °C.[2] It shows a light blue fluorescence. It is very slightly soluble in water, and soluble in ethanol and propylene glycol. It is insoluble in paraffin oil. It is combustible, with flash point at 104 °C. At full concentration, it has a fruity grape smell; at 25 ppm it has a sweet, fruity, Concord grape-like smell with a musty and berry nuance.[3]
Uses
Methyl anthranilate acts as a bird repellent. It is food-grade and can be used to protect corn, sunflowers, rice, fruit, and golf courses. Dimethyl anthranilate (DMA) has a similar effect. It is also used for the flavor of grape Kool-Aid. It is used for flavoring of candy, soft drinks , e.g. grape soda, chewing gum, drugs and, nicotine products.[4]
Methyl anthranilate both as a component of various natural essential oils and as a synthesised aroma-chemical is used extensively in modern perfumery.[3][5] It is also used to produce Schiff bases with aldehydes, many of which are also used in perfumery. In a perfumery context the most common Schiff's Base is known as aurantiol,[6] produced by combining methyl anthranilate and hydroxycitronellal.[7]
Occurrence
Methyl anthranilate naturally occurs in the Concord grapes and other Vitis labrusca grapes and hybrids thereof, and in bergamot, black locust, champak, gardenia, jasmine, lemon, mandarin orange, neroli, oranges, rue oil, strawberry, tuberose, wisteria, galangal, and ylang ylang. It is also a primary component of the essential apple flavor, along with ethyl acetate and ethyl butyrate.[8]
References
- ↑ Kováts, Kugler (1963). "Zur Kenntnis ätherischer Öle. 1. Mitteilung. Zur Kenntnis des Mandarinenschalen-Öls (Citrus reticulata BLANCO, bzw. Citrus nobilis var. deliciosa SWINGLE "Mandarin")". Helvetica Chimica Acta. 46 (5): 1480–1513.
- ↑ Halama; Pytela (1996). "Steric Effects in Acid-Catalyzed Decomposition and Base-Catalyzed Cyclization of 1-(2-Alkoxycarbonylphenyl)-3-phenyltriazenes". Collection of Czechoslovak Chemical Communications. 61 (5): 751.
- 1 2 The Good Scents Company: Methyl anthranilate
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMc1403015
- ↑ An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN 978-0-9608752-8-3, ISBN 978-1-870228-24-4
- ↑ The Chemistry of Fragrances: From Perfumer to Consumer, ed. Charles Sell, ISBN 0-85404-824-3, ISBN 978-085404-824-3
- ↑ Good Scents Company Page for Aurantiol
- ↑ Daniele Fraternale, Donata Ricci, Guido Flamini and Giovanna Giomaro. Volatiles Profile of Red Apple from Marche Region (Italy). Rec. Nat. Prod. (2011), 5:3; 202-207. pdf