Methylammonium lead halide
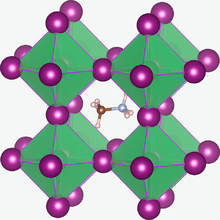
Methylammonium lead halides (MALHs) are solid compounds with perovskite structure and a chemical formula of CH3NH3PbX3 (MAPbX3), where X = I, Br or Cl. They have potential applications in solar cells, lasers, light-emitting diodes, photodetectors, radiation detectors [2][3] magneto-optical data storage[4] and hydrogen production.[5]
Properties and synthesis
In the CH3NH3PbX3 crystal structure the methylammonium cation (CH3NH3+) is surrounded by PbX6 octahedra. The X ions are not fixed and can migrate through the crystal with an activation energy of 0.6 eV; the migration is vacancy assisted.[1] The methylammonium cations can rotate within their cages. At room temperature the ions have the CN axis aligned towards the face directions of the unit cells and the molecules randomly change to another of the six face directions on a 3 ps time scale.[6]


The solubility of MALHs strongly decreases with temperature: from 0.8 g/mL at 20 °C to 0.3 g/mL at 80 °C for CH3NH3PbI3 in dimethylformamide. This property is used in the growth of MALH single crystals and films from solution, using a mixture of CH3NH3X and PbX2 powders as the precursor. The growth rates are 3–20 mm3/hour for CH3NH3PbI3 and reach 38 mm3/hour for CH3NH3PbBr3 crystals.[5]
The resulting crystals are metastable and dissolve in the growth solution when cooled to room temperature. They have bandgaps of 2.18 eV for CH3NH3PbBr3 and 1.51 eV for CH3NH3PbI3, while their respective carrier mobilities are 24 and 67 V/(cm2·s).[5] Their thermal conductivity is exceptionally low, ~0.5 W/(K·m) at room temperature for CH3NH3PbI3.[7]
Applications
MALHs have potential applications in solar cells, lasers, light-emitting diodes, photodetectors, radiation detectors [3] and hydrogen production.[5] The power conversion efficiency of MALH solar cells exceeds 19%.[8][9]
See also
References
| Wikimedia Commons has media related to Methylammonium lead halides. |
- 1 2 Eames, Christopher; Frost, Jarvist M.; Barnes, Piers R. F.; o'Regan, Brian C.; Walsh, Aron; Islam, M. Saiful (2015). "Ionic transport in hybrid lead iodide perovskite solar cells". Nature Communications. 6: 7497. doi:10.1038/ncomms8497. PMC 4491179
 . PMID 26105623.
. PMID 26105623. - ↑ Náfrádi, Bálint (October 16, 2015). "Methylammonium Lead Iodide for Efficient X-ray Energy Conversion". J. Phys. Chem. C. 2015 (119): 25204–25208. doi:10.1021/acs.jpcc.5b07876.
- 1 2 Yakunin, S.; Dirin, D.; Shynkarenko, Y.; Morad, V.; Cherniukh, I.; Nazarenko, O.; Kreil, D.; Nauser, T.; Kovalenko, M. (2016). "Detection of gamma photons using solution-grown single crystals of hybrid lead halide perovskites". Nature Photonics. 10: 585–589. doi:10.1038/nphoton.2016.139.
- ↑ Náfrádi, Bálint (24 November 2016). "Optically switched magnetism in photovoltaic perovskite CH3NH3(Mn:Pb)I3". Nature Communications. 7: 13406. doi:10.1038/ncomms13406.
- 1 2 3 4 5 6 Saidaminov, Makhsud I.; Abdelhady, Ahmed L.; Murali, Banavoth; Alarousu, Erkki; Burlakov, Victor M.; Peng, Wei; Dursun, Ibrahim; Wang, Lingfei; He, Yao; MacUlan, Giacomo; Goriely, Alain; Wu, Tom; Mohammed, Omar F.; Bakr, Osman M. (2015). "High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization". Nature Communications. 6: 7586. doi:10.1038/ncomms8586. PMC 4544059
 . PMID 26145157.
. PMID 26145157. - ↑ Bakulin, A.A.; Selig, O.; Bakker, H.J.; Rezus, Y.L.A.; Muller, C.; Glaser, T.; Lovrincic, R.; Sun, Z.; Chen, Z.; Walsh, A.; Frost, J.M.; Jansen, T.L.C. (2015). "Real-Time Observation of Organic Cation Reorientation in Methylammonium Lead Iodide Perovskites". J. Phys. Chem. Lett. 6 (18): 3663–3669. doi:10.1021/acs.jpclett.5b01555.
- ↑ Pisoni, Andrea; Jaćimović, Jaćim; Barišić, Osor S.; Spina, Massimo; Gaál, Richard; Forró, László; Horváth, Endre (2014). "Ultra-Low Thermal Conductivity in Organic–Inorganic Hybrid Perovskite CH3NH3PbI3". The Journal of Physical Chemistry Letters. 5 (14): 2488–2492. doi:10.1021/jz5012109. PMID 26277821.
- ↑ Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. (2014). "Interface engineering of highly efficient perovskite solar cells". Science. 345 (6196): 542. doi:10.1126/science.1254050. PMID 25082698.
- ↑ Heo, Jin Hyuck; Song, Dae Ho; Han, Hye Ji; Kim, Seong Yeon; Kim, Jun Ho; Kim, Dasom; Shin, Hee Won; Ahn, Tae Kyu; Wolf, Christoph; Lee, Tae-Woo; Im, Sang Hyuk (2015). "Planar CH3NH3PbI3 Perovskite Solar Cells with Constant 17.2% Average Power Conversion Efficiency Irrespective of the Scan Rate". Advanced Materials. 27 (22): 3424. doi:10.1002/adma.201500048. PMID 25914242.