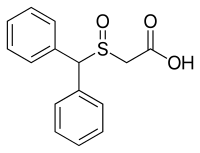
Modafinil acid
 | |
| Names | |
|---|---|
| IUPAC name
2-Benzhydrylsulfinylacetic acid | |
| Other names
Modafinilic acid; Modafinil carboxylate; CRL-40467 | |
| Identifiers | |
| 63547-24-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 2342211 |
| PubChem | 3085267 |
| UNII | 54N37HN7N4 |
| |
| |
| Properties | |
| C15H14O3S | |
| Molar mass | 274.33 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Modafinil acid (code name CRL-40467), also known as modafinilic acid or modafinil carboxylate, is the major metabolite of modafinil, and one of the two major metabolites of modafinil – the other being modafinil sulfone. Modafinil acid is also a metabolite of the modafinil prodrug, adrafinil, and the (R)-(–)-enantiomer is a metabolite of armodafinil, the (R)-(–)-enantiomer of modafinil. Modafinil acid seems to be inactive,[1] and similarly to modafinil sulfone, does not appear to contribute to the wakefulness-promoting/psychostimulant effects of modafinil.[2][3][4]
In the breakdown process of modafinil, modafinil is primarily hydrolyzed by an esterase or amidase enzyme into modafinil acid.[5] The apparent clearance of modafinil acid is significantly higher than that of modafinil, following the hypothesis that metabolism increases the polarity and the clearance of modafinil.
References
- ↑ Wong, Y. Nancy; Wang, Lixia; Hartman, Linda; Simcoe, Donna; Chen, Yusong; Laughton, Watson; Eldon, Richard; Markland, Colin; Grebow, Peter (1998). "Comparison of the Single-Dose Pharmacokinetics and Tolerability of Modafinil and Dextroamphetamine Administered Alone or in Combination in Healthy Male Volunteers". The Journal of Clinical Pharmacology. 38 (10): 971–978. doi:10.1002/j.1552-4604.1998.tb04395.x. ISSN 0091-2700.
- ↑ Schwertner, Harvey A.; Kong, Suk Bin (2005). "Determination of modafinil in plasma and urine by reversed phase high-performance liquid-chromatography". Journal of Pharmaceutical and Biomedical Analysis. 37 (3): 475–479. doi:10.1016/j.jpba.2004.11.014. ISSN 0731-7085.
- ↑ Robertson, Philmore; Hellriegel, Edward T. (2003). "Clinical Pharmacokinetic Profile of Modafinil". Clinical Pharmacokinetics. 42 (2): 123–137. doi:10.2165/00003088-200342020-00002. ISSN 0312-5963.
- ↑ Robertson, P (2002). "Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers". Clinical Pharmacology & Therapeutics. 71 (1): 46–56. doi:10.1067/mcp.2002.121217. ISSN 0009-9236.
- ↑ Wu, Ke-hua; Guo, Tao; Deng, Chen-hui; Guan, Zheng; Li, Liang; Zhou, Tian-yan; Lu, Wei (2012). "Population pharmacokinetics of modafinil acid and estimation of the metabolic conversion of modafinil into modafinil acid in 5 major ethnic groups of China". Acta Pharmacologica Sinica. 33 (11): 1401–1408. doi:10.1038/aps.2012.124. ISSN 1671-4083.