Naphthalenetetracarboxylic dianhydride
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Isochromeno[6,5,4-def]isochromene-1,3,6,8-tetrone | |
| Other names
1,4,5,8-Naphthalenetetracarboxylic dianhydride | |
| Identifiers | |
| 81-30-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 6426 |
| ECHA InfoCard | 100.001.221 |
| PubChem | 24897857 |
| |
| Properties | |
| C14H4O6 | |
| Molar mass | 268.18 g·mol−1 |
| Appearance | Beige powder |
| Melting point | > 300 °C (572 °F; 573 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Napthalenetetracarboxylic dianhydride is an organic compound related to naphthalene. The compound is a beige solid. NTDAs are most commonly used as a precursor to naphthalenediimides (NDIs), a family of compound with many different uses.[1]
Synthesis and structure
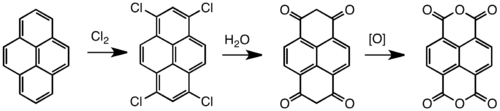
Naphthalene dianhydride is prepared by oxidation of pyrene. Typical oxidants are chromic acid and chlorine. The unsaturated tetrachloride hydrolyzes to enols that tautomerize to the bis-dione, which in turn can be oxidized to the tetracarboxylic acid.[2]
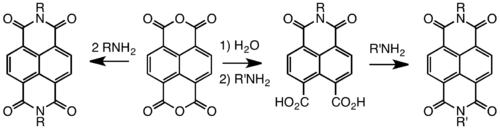
Naphthalene diimides
Symmetrical naphthalene diimides are synthesized by the condensation reaction of primary amines and the dianhydride. Unsymmetrical derivatives, i.e. those derived from two different amines, are obtained by hydrolysis of one of the two anhydride groups prior to the condensation with the first amine.
These diimides are members of a broader class of compounds called rylenes, oligomers of naphthalene with bonds between the 1 and 1' and 8 and 8' positions. The resulting materials have rigidly planar, highly conjugated cores. They exhibit good processing characteristics for fabrication of soft electronic devices. Aside from the NDI's, other members include the diimide derivatives of perylene-3,4:9,10-tetracarboxylic dianhydride and terrylene-3,4:11,12-tetracarboxylic dianhydride.[4]
Naphthalene diimides (NDIs) are often fluorescent, although the intensity is sensitive to substituents. NDIs are also redox-active, forming stable radical anions near -1.10 V vs. Fc/Fc+.[1] Their ability to accept electrons reflects the presence of an extended conjugated ring system and the electron withdrawing groups (carbonyl centers). NDIs are used ins supramolecular chemistry due to their tendency to form charge-transfer complexes with crown ethers, e.g. to give rotaxanes and catenanes. As another consequence of their planar structure and electron-acceptor properties, NDIs intercalate into DNA.
Because a range of amines can be condensed with the dianhydride. For example, two useful pigments of the perinone class are generated by condensation with phenylenediamine. A variety of ligands with NDI backbones have also been prepared.[5]
References
- 1 2 Bhosale, S.; Jani, C.; Langford, S. "Chemistry of Naphthalene Diimides" Chem. Soc. Rev., 2008, 37, 331-342. doi:10.1039/b615857a
- ↑ F. Röhrscheid "Carboxylic Acids, Aromatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a05_249
- ↑ Mikio Yasutake, Takashi Fujihara, Akira Nagasawa, Keiichi Moriya, Takuji Hirose "Synthesis and Phase Structures of Novel π-Acceptor Discotic Liquid Crystalline Compounds Having a Pyrenedione Core" European Journal of Organic Chemistry 2008, volume 24, 4120-4125. doi:10.1002/ejoc.200800360
- ↑ X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, "Rylene and Related Diimides for Organic Electronics", Advanced Materials 2011, volume 23, pp 268-284. doi:10.1002/adma.201001402
- ↑ Mei Pan, Xiao-Ming Lin, Guo-Bi Li, Cheng-Yong Su "Progress in the study of metal–organic materials applying naphthalene diimide (NDI) ligands" Coordination Chemistry Reviews 2011, volume 255, 1921–1936. doi:10.1016/j.ccr.2011.03.013