Niementowski quinazoline synthesis
| Niementowski quinazoline synthesis | |
|---|---|
| Named after | Stefan Niementowski |
| Reaction type | Ring forming reaction |
The Niementowski quinazoline synthesis is the chemical reaction of anthranilic acids with amides to form 4-oxo-3,4-dihydroquinazolines (3H-quinazolin-4-ones).

Uses
Research has demonstrated that the Niementowski quinazoline synthesis could be employed for the creation of potential EGFR-inhibiting molecules. Hensbergen et al.[1] have shown a synthetic route to a new class of privileged tri- and tetra-cyclic quinazolines containing a medium-sized ring.
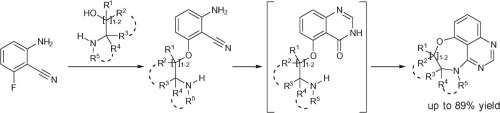
A nucleophilic aromatic substitution is combined with the Niementowski reaction and a BOP-mediated ring closure to afford several analogues.
References
- ↑ Hensbergen, Albertus Wijnand; Mills, Vanessa R.; Collins, Ian; Jones, Alan M. (18 November 2015). "An expedient synthesis of oxazepino and oxazocino quinazolines". Tetrahedron Letters. doi:10.1016/j.tetlet.2015.10.008.
- ^ Niementowski, Stefan (1895). "Synthesen von Chinazolinverbindungen". J. Prakt. Chem. 51: 564.
- ^ Williamson, T. A. (1957). "The chemistry of quinazoline". Heterocyclic Compounds. 6: 331.
- ^ Cuny, Eckehard; Lichtenthaler, F.W.; Moser, Alfred (1980). "Benzologs of allopurinol: Synthesis of pyrazolo [4,3-g] and [3,4-f] quinazolinones". Tetrahedron Lett. 21 (32): 3029. doi:10.1016/S0040-4039(00)77398-9.
This article is issued from Wikipedia - version of the 6/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.