Nucleoside-phosphate kinase
| nucleoside phosphate kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.7.4.4 | ||||||||
| CAS number | 9026-50-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, a nucleoside-phosphate kinase (EC 2.7.4.4) is an enzyme that catalyzes the chemical reaction[1]
- ATP + nucleoside phosphate ADP + nucleoside diphosphate
Thus, the two substrates of this enzyme are ATP and nucleoside monophosphate, whereas its two products are ADP and nucleoside diphosphate.[2][3]
This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with a phosphate group as acceptor.[4] The systematic name of this enzyme class is ATP:nucleoside-phosphate phosphotransferase. This enzyme is also called NMP-kinase, or nucleoside-monophosphate kinase.
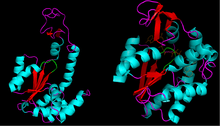
Structure
A number of crystal structures have been solved for this class of enzymes, revealing that they share a common ATP binding domain. This section of the enzyme is commonly referred to as the P-loop,[5] in reference to its interaction with the phosphoryl groups on ATP. This binding domain also consists of a β sheet flanked by α helices.
The [P-loop] typically has the amino acid sequence of Gly-X-X-X-X-Gly-Lys.[6] Similar sequences are found in many other nucleotide-binding proteins.
Mechanism
Metal Ion Interaction
To allow for interaction with this class of enzymes, ATP must first bind to a metal ion such as magnesium or manganese.[8] The metal ion forms a complex with the phosphoryl-group, as well as several water molecules.[9] These water molecules then form hydrogen bonds to a conserved aspartate residue on the enzyme.[10]
The metal ion interaction facilitates binding by holding the ATP molecule in a position allowing for specific binding to the active site and by providing additional points for binding between the substrate and the enzyme. This increases the binding energy.
Conformational Changes
Binding of ATP causes the P-loop to move, in turn making the lid domain lower and secure the ATP in place.[11][12] Nucleoside monophosphate binding induces further changes that render the enzyme catalytically capable of facilitating a transfer of the phosphoryl group from ATP to nucleoside monophosphate.[13]
The necessity of these conformational changes prevents the wasteful hydrolysis of ATP.
This enzyme mechanism is an example of catalysis by approximation: the nucleoside-phosphate kinase binds the substrates to bring them together in the correct position for the phosphoryl group to be transferred.
Biological Function
Similar catalytic domains are present in a variety of proteins, including:
- ATP synthase
- Myosin, and other molecular motor proteins
- G protein and other proteins involved in signal transduction
- Helicases for unwinding DNA and RNA
- Pyrimidine metabolism
Evolution
When a phylogenetic tree composed of members of the nucleoside-phosphate kinase family was made,[14] it showed that these enzymes had originally diverged from a common ancestor into long and short varieties. This first change was drastic – the three-dimensional structure of the lid domain changed significantly.
Following the evolution of long and short varieties of NMP-kinases, smaller changes in the amino acid sequences resulted in the differentiation of subcellular localization.
References
- ↑ Boyer, P.D., Lardy, H. and Myrback, K. (Eds.), The Enzymes, 2nd ed., vol. 6, Academic Press, New York, 1962, p. 139-149.
- ↑ AYENGAR P, GIBSON DM, SANADI DR (1956). "Transphosphorylations between nucleoside phosphates". Biochim. Biophys. Acta. 21 (1): 86–91. doi:10.1016/0006-3002(56)90096-8. PMID 13363863.
- ↑ LIEBERMAN I, KORNBERG A, SIMMS ES (1955). "Enzymatic synthesis of nucleoside diphosphates and triphosphates". J. Biol. Chem. 215 (1): 429–40. PMID 14392176.
- ↑ HEPPEL LA, STROMINGER JL, MAXWELL ES (1959). "Nucleoside monophosphate kinases. II. Transphosphorylation between adenosine monophosphate and nucleoside triphosphates". Biochim. Biophys. Acta. 32: 422–30. doi:10.1016/0006-3002(59)90615-8. PMID 14401179.
- ↑ Dreusicke, D.; Schulz, G.E. (1986). "The glycine-rich loop of adenylate kinase forms a giant anion hole". FEBS Lett. 208 (2): 301–304. doi:10.1016/0014-5793(86)81037-7. PMID 3023140.
- ↑ Byeon, L.; Shi, Z.; Tsai, M.D. (1995). "Mechanism of adenylate kinase. The "essential lysine" helps to orient the phosphates and the active site residues to proper conformations". Biochemistry. 34 = (10): 3172–3182. doi:10.1021/bi00010a006. PMID 7880812.
- ↑ Muller, C.W.; Schlauderer, G.J.; Reinstein, J.; Schulz, G.E. (1996). "Adenylate kinase motions during catalysis: an energetic counterweight balancing substrate binding.". Structure. 4 (2): 147–156. doi:10.2210/pdb4ake/pdb. PMID 8805521.
- ↑ Berg, J.M.; Tymoczko, J.L.; Stryer, L. (2002). Biochemistry. New York: W H Freeman. ISBN 0-7167-3051-0. Retrieved 2016-01-08.
- ↑ Krishnamurthy, H.; Lou, H.; Kimple, A.; Vieille, C.; Cukier, R.I. (2005). "Associative mechanism for phosphoryl transfer: a molecular dynamics simulation of Escherichia coli adenylate kinase complexed with its substrates". Proteins. 58 (1): 88–100. doi:10.1002/prot.20301. PMID 15521058.
- ↑ Pai, E.F.; Sachseneheimer, W.; Schirmer, R.H.; Schulz, G.E. (1996). "Substrate positions and induced-fit in crystalline adenylate kinase". J. Mol. Biol. 114 (1): 37–45. doi:10.1016/0022-2836(77)90281-9. PMID 198550.
- ↑ Muller, C.W.; Schulz, G.E. (1992). "Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A at 1.9 A resolution. A model for a catalytic transition state.". J.Mol.Biol. 224 (1): 159–177. doi:10.2210/pdb1ake/pdb. PMID 1548697.
- ↑ Schlauderer, G.J.; Proba, K.; Schulz, G.E. (1996). "Structure of a mutant adenylate kinase ligated with an ATP-analogue showing domain closure over ATP". J. Mol. Biol. 256 (2): 223–227. doi:10.1006/jmbi.1996.0080. PMID 8594191.
- ↑ Vonrhein, C.; Schlauderer, G.J.; Schulz, G.E. (1995). "Movie of the structural changes during a catalytic cycle of nucleoside monophosphate kinases". Structure. 3 (5): 483–490. doi:10.1016/s0969-2126(01)00181-2. PMID 7663945.
- ↑ Fukami-Kobayashi, Kaoru; Nosaka, Michiko; Nakazawa, Atsushi; Gō, Mitiko (1996). "Ancient Divergence of long and short isoforms of adenylate kinase molecular evolution of the nucleoside monophosphate kinase family". FEBS Letters. 385 (3): 214–220. doi:10.1016/0014-5793(96)00367-5. PMID 8647254.