Opsonin
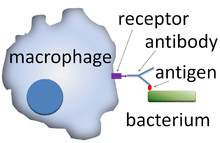
An opsonin (from the Greek opsōneîn, to prepare for eating) is any molecule that enhances phagocytosis by marking an antigen for an immune response or marking dead cells for recycling (i.e., causes the phagocyte to "relish" the marked cell).[1] Opson in ancient Greece referred to the delicious side-dish of any meal, versus the sitos, or the staple of the meal.
Opsonization (also, opsonisation) is the molecular mechanism whereby molecules, microbes, or apoptotic cells are chemically modified to have stronger interactions with - to be more "delicious" to - cell surface receptors on phagocytes and NK cells. With the antigen coated in opsonins, binding to immune cells is greatly enhanced. Opsonization also mediates phagocytosis via signal cascades from cell surface receptors.[2]
Opsonins aid the immune system in a number of ways. In a healthy individual, they mark dead and dying self cells for clearance by macrophages and neutrophils, activate complement proteins, and target cells for destruction through the action of natural killer (NK) cells.
Mechanism
All cell membranes have negative charges (Zeta potential) which makes it difficult for two cells to come close together. When opsonins bind to their targets they boost the kinetics of phagocytosis by favoring interaction between the opsonin and cell surface receptors on immune cells.[3] This overrides the negative charges from cell membranes. This principal holds true for clearance of pathogens as well as dead or dying self cells.
Varieties
Different opsonins perform different functions. Opsonin molecules include:
Antibodies
Antibodies are part of the adaptive immune response and are generated by B cells in response to antigen exposure. The Fab region of the antibody binds to the antigen, whereas the Fc region of the antibody binds to an Fc receptor on the phagocyte, facilitating phagocytosis.[4] The antigen-antibody complex can also activate complement through the classical complement pathway. Phagocytic cells do not have an Fc receptor for immunoglobulin M (IgM),[5] making IgM ineffective in assisting phagocytosis alone. However, IgM is extremely efficient at activating complement[6] and is, therefore, considered an opsonin.[7] IgG antibodies are also capable of binding immune effector cells via their Fc domain, triggering a release of lysis products from the bound immune effector cell (monocytes, neutrophils, eosinophils, and natural killer cells). This process, called antibody-dependent cellular cytotoxicity, can cause inflammation of surrounding tissues and damage to healthy cells.
Complement proteins
The complement system is a part of the innate immune response. C3b, C4b, and C1q are important complement molecules that serve as opsonins. As a part of the alternative complement pathway, the spontaneous activation of a complement cascade converts C3 to C3b, a component that can serve as an opsonin when bound to an antigen's surface.[8] Antibodies can also activate complement via the classical pathway, resulting in deposition of C3b and C4b onto the antigen surface.[9] After C3b has bound to the surface of an antigen, it can be recognized by phagocyte receptors that signal for phagocytosis. Complement receptor 1 is expressed on all phagocytes and recognizes a number of complement opsonins, including C3b[9] and C4b which are both parts of C3-convertase. C1q, a member of the C1 complex, is able to interact with the Fc region of antibodies.[9]
Circulating proteins
Pentraxins, collectins, and ficolins are all circulating proteins that are capable of serving as opsonins.[10] They are secreted Pattern recognition receptors (PRRs). These molecules coat the microbes as opsonins and enhance neutrophil reactivity against them through a number of mechanisms.
Targets
Apoptotic cells
Apoptosis is related to low tissue inflammation. A number of opsonins play a role in marking apoptotic cells for phagocytosis without a pro-inflammatory response.[10]
Members of the pentraxin family can bind to apoptotic cell membrane components like phosphatidylcholine (PC) and phosphatidylethanolamine (PE). IgM antibodies also bind to PC. Collectin molecules such as mannose-binding lectin (MBL), surfactant protein A (SP-A), and SP-D interact with unknown ligands on apoptotic cell membranes. When bound to the appropriate ligand these molecules interact with phagocyte receptors, enhancing phagocytosis of the marked cell.[3]
C1q is capable of binding directly to apoptotic cells. It can also indirectly bind to apoptotic cells via intermediates like IgM autoantibodies, MBL, and pentraxins. In both cases C1q activates complement, resulting in the cells being marked for phagocytosis by C3b and C4b. C1q is an important contributor to the clearance of apoptotic cells and debris. This process usually occurs in late apoptotic cells.[3]
Opsonization of apoptotic cells occurs by different mechanisms in a tissue-dependent pattern. For example, while C1q is necessary for proper apoptotic cell clearance in the peritoneal cavity, it is not important in the lungs where SP-D plays an important role.[3]
Pathogens
As part of the late stage adaptive immune response, pathogens and other particles are marked by IgG antibodies. These antibodies interact with Fc receptors on macrophages and neutrophils resulting in phagocytosis.[2] The C1 complement complex can also interact with the Fc region of IgG and IgM immune complexes activating the classical complement pathway and marking the antigen with C3b. C3b can spontaneously bind to pathogen surfaces through the alternative complement pathway. Furthermore, pentraxins can directly bind to C1q from the C1 complex.[9]
SP-A opsonizes a number of bacterial and viral pathogens for clearance by lung alveolar macrophages.[10]
See also
References
- ↑ Barret, James (1980). Basic Immunology and its Medical Application (2 ed.). St.Louis: The C.V. Mosby Company. ISBN 0-8016-0495-8.
- 1 2 Zhang, Youxin; Hoppe, Adam D.; Swanson, Joel A. (2010-11-09). "Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis". Proceedings of the National Academy of Sciences. 107 (45): 19332–19337. doi:10.1073/pnas.1008248107. ISSN 0027-8424. PMC 2984174
 . PMID 20974965.
. PMID 20974965. - 1 2 3 4 Roos, Anja; Xu, Wei; Castellano, Giuseppe; Nauta, Alma J.; Garred, Peter; Daha, Mohamed R.; van Kooten, Cees (2004-04-01). "Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells". European Journal of Immunology. 34 (4): 921–929. doi:10.1002/eji.200424904. ISSN 1521-4141.
- ↑ Parham, P. (2005). The Immune System," Garland Science Publishing, New York, NY.
- ↑ Shima, Hideaki; Hiroyuki, Takatsu (2009). "Identification of TOSO/FAIM3 as an Fc receptor for IgM". International Immunology. Oxford Journals. 22 (3): 149–56. doi:10.1093/intimm/dxp121. Retrieved 30 November 2014.
- ↑ Shulman, MJ; Pennel, N (1986). "Activation of complement by immunoglobulin M is impaired by the substitution serine-406----asparagine in the immunoglobulin mu heavy chain" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 83 (20): 7678–82. doi:10.1073/pnas.83.20.7678. PMID 3094013. Retrieved 30 November 2014.
- ↑ Williams, MR; Hill, AW (1982). "A role for IgM in the in vitro opsonisation of Staphylococcus aureus and Escherichia coli by bovine polymorphonuclear leucocytes". Research in Veterinary Science. Elsevier. 33 (1): 47–53. PMID 6753075. Retrieved 30 November 2014.
- ↑ Tosi, Michael F. (2005-08-01). "Innate immune responses to infection". Journal of Allergy and Clinical Immunology. 116 (2): 241–249. doi:10.1016/j.jaci.2005.05.036.
- 1 2 3 4 Sarma, J. Vidya; Ward, Peter A. (2010-09-14). "The complement system". Cell and Tissue Research. 343 (1): 227–235. doi:10.1007/s00441-010-1034-0. ISSN 0302-766X. PMC 3097465
 . PMID 20838815.
. PMID 20838815. - 1 2 3 Litvack, Michael L.; Palaniyar, Nades (2010-06-01). "Review: Soluble innate immune pattern-recognition proteins for clearing dying cells and cellular components: implications on exacerbating or resolving inflammation". Innate Immunity. 16 (3): 191–200. doi:10.1177/1753425910369271. ISSN 1753-4259. PMID 20529971.
External links
- Opsonins at the US National Library of Medicine Medical Subject Headings (MeSH)