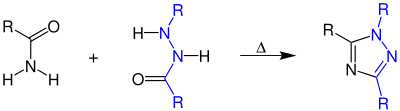
Pellizzari reaction
The Pellizzari reaction was discovered in 1911 by Guido Pellizzari, and is the organic reaction of an amide and a hydrazide to form a 1,2,4-triazole.[1]
The product is similar to that of the Einhorn-Brunner reaction, but the mechanism itself is not regioselective.
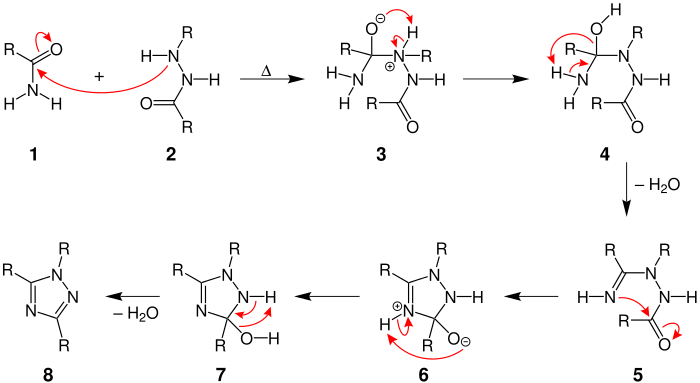
Mechanism
The mechanism begins by the nitrogen in the hydrazide attacking the carbonyl carbon on the amide to form compound 3. The negatively charged oxygen then abstracts two hydrogens from neighboring nitrogens in order for a molecule of water to be released to form compound 5. The nitrogen then performs an intramoleculer attack on the carbonyl group to form the five-membered ring of compound 6. After another proton migration from the nitrogens to the oxygen, another water molecule is released to form the 1,2,4-triazole 8. [2]
Uses
The synthesis of the 1,2,4-triazole has a wide range of biological functions.[3] 1,2,4-triazoles have antibacterial, antifungal, antidepressant and hypoglycemic properties. 3-benzylsulfanyl derivates of the triazole also show slight to moderate antimycobacterial activity, but are considered moderately toxic. [4]
Problems and variations
The Pellizzari is limited in the number of substituents that can be on the ring, so other methods have been developed to incorporate three elements of diversity. Liquid-phase synthesis of 3-alkylamino-4,5-disubstituted-1,2,4-triazoles via PEG support has given moderate yields with excellent purity.[5] In practice, the Pellizzari reaction requires high temperatures, long reaction times, and has an overall low yield. However, adding microwave irradiation shortens the reaction time and increases its yield.[6]
References
- ↑ Pellizzari, G. Gazz. Chim. Ital. 1911, 41, 20.
- ↑ Wang, Z (2009). Comprehensive Organic Name Reactions. 2. John Wiley & Sons. pp. 2157–2158. ISBN 9780471704508.
- ↑ Gadhave, Priyanka; Dighe, Nachiket (2010). "Current biological and synthetic profile of Triazoles: A review" (PDF). Annals of Biological Research. 1 (1): 82–89.
- ↑ Klimesová, V.; Zahajská, L. (2004). "Synthesis and antimycobacterial activity of 1,2,4-triazole 3-benzylsulfanyl derivatives.". Il Farmaco. 4: 279–288.
- ↑ Zong, Ying-Xiao; Wang, Jun-Ke; Yue, Guo-Ren; Feng, Lei; Song, Zheng-En; Song, Hai; Han, Yu-Qi (2005). "Traceless liquid-phase synthesis of 3-alkylamino-4,5-disubstituted-1,2,4-triazoles on polyethylene glycol (PEG)". Tetrahedron Letters. 46 (31): 5139–5141. doi:10.1016/j.tetlet.2005.05.121.
- ↑ Lee, Jongbok; Hong, Myengchan; Jung, Yoonchul; Cho, Eun Jin; Rhee, Hakjune (2012). "Synthesis of 1,3,5-trisubstituted-1,2,4-triazoles by microwave-assisted N-acylation of amide derivatives and the consecutive reaction with hydrazine hydrochlorides" (PDF). Tetrahedron. 68: 2045–2051. doi:10.1016/j.tet.2012.01.003.