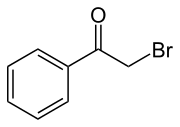
Phenacyl bromide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Bromo-1-phenylethanone | |
| Other names
2-Bromoacetophenone; α-Bromoacetophenone; Bromomethyl phenyl ketone | |
| Identifiers | |
| 70-11-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:51846 |
| ChEMBL | ChEMBL102953 |
| ChemSpider | 6023 |
| ECHA InfoCard | 100.000.659 |
| PubChem | 6259 |
| |
| |
| Properties | |
| C8H7BrO | |
| Molar mass | 199.05 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 50 °C (122 °F; 323 K)[1] |
| Boiling point | 136 °C (277 °F; 409 K) 18 mm Hg[1] |
| Hazards | |
| Main hazards | Toxic(T) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phenacyl bromide is the organic compound with the formula C6H5C(O)CH2Br. This colourless solid is a powerful lachrymator as well as a useful precursor to other organic compounds.
It is prepared by bromination of acetophenone:[2]
- C6H5C(O)CH3 + Br2 → C6H5C(O)CH2Br + HBr
The compound was first reported in 1871.[3]
References
- 1 2 Phenacyl Bromide, TCI America
- ↑ R. M. Cowper and L. H. Davidson. "Phenacyl bromide". Org. Synth.; Coll. Vol., 2, p. 480
- ↑ A. Emmerling and C. Engler (1871). "Ueber einige Abkömmlinge des Acetophenons". Ber. 4 (1): 147–149. doi:10.1002/cber.18710040149.
This article is issued from Wikipedia - version of the 7/16/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.