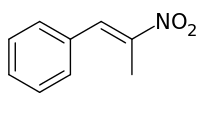
Phenyl-2-nitropropene
 | |
| Names | |
|---|---|
| IUPAC name
1-Phenyl-2-nitropropene | |
| Other names
P2NP, β-methyl-β-nitropropene, (2-Nitro-1-propenyl)benzene | |
| Identifiers | |
| 705-60-2 [1] | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 1266396 |
| ECHA InfoCard | 100.155.731 |
| PubChem | 1549520 |
| |
| |
| Properties | |
| C9H9NO2 | |
| Molar mass | 163.17 g mol−1 |
| Appearance | solid |
| Melting point | 64 to 66 °C (147 to 151 °F; 337 to 339 K) |
| Hazards | |
| EU classification (DSD) |
Harmful (Xn) |
| R-phrases | R22, R36/37/38 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
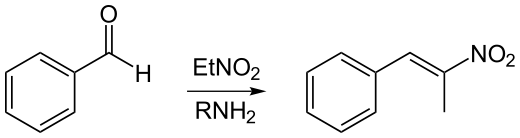
Phenyl-2-nitropropene is a chemical compound with the chemical formula is C9H9NO2. It can be produced by the reaction of benzaldehyde and nitroethane in the presence of a basic catalyst. In this reaction, the base deprotonates nitroethane to form a resonance stabilized anion. This anion nucleophilically adds to the aldehyde forming a beta nitro alcohol, which is subsequently dehydrated to yield the nitroalkene. This reaction is known as a nitroaldol reaction. Phenyl-2-nitropropene can be reduced in the presence of a catalyst to produce phenylacetone, which is a controlled precursor of methamphetamine. However, with lithium aluminium hydride it can simply be reduced to directly form amphetamine in moderate yields.
References
This article is issued from Wikipedia - version of the 5/19/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.