Piscarinine
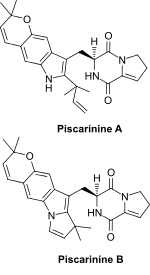
2D Structure of Piscarinine A and Piscarinine B
Piscarinines are bioactive alkaloid isolates of Penicillium piscarium NKM F-961 [1] and Penicillium piscarium Westling [2] that belong to a class of naturally occurring 2,5-diketopiperazines.[3] The cytotoxic dehydroproline tryptophan derivatives piscarinines A and B were shown to be active against the prostate cancer cell line LNCAP [2] (IC50 values were 2.2 and 1.9 μg/mL for piscarinine A and B, respectively).
References
- ↑ Kozlovsky A, Vinokurova NG, Adanin VM, Gräfe U (September 2000). "Piscarinines, new polycyclic diketopiperazine alkaloids from Penicillium piscarium NKM F-691". Natural Product Letters. 14 (5): 333–340. doi:10.1080/10575630008043765.
- 1 2 Zhelifonova VP, Maier A, Kozlovskii AG (November 2008). "Effect of various factors on the biosynthesis of piscarinines, secondary metabolites of the fungus Penicillium piscarium Westling". Applied biochemistry and microbiology. 44 (6): 608–612. doi:10.1134/S0003683808060082. PMID 19145974.
- ↑ Borthwick AD (May 2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews. 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.