Procyanidin B1
 | |
| Names | |
|---|---|
| IUPAC name
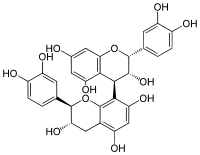
(2R,2ʼR,3R,3ʼS,4R)-2,2ʼ-bis(3,4-dihydroxyphenyl)-3,3ʼ,4,4ʼ-tetrahydro-2H,2ʼH-4,8ʼ-bichromene-3,3ʼ,5,5ʼ,7,7ʼ-hexol | |
| Other names
Procyanidin B1 cis,trans′′-4,8′′-Bi-(3,3′,4′,5,7-Pentahydroxyflavane) | |
| Identifiers | |
| 20315-25-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75633 |
| ChEMBL | ChEMBL504937 |
| ChemSpider | 9425166 |
| PubChem | 11250133 |
| UNII | 0566J48E7X |
| |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Procyanidin B1[1] is a procyanidin dimer.
It is a molecule with a 4→8 bond (epicatechin-(4β→8)-catechin).[2] Proanthocyanidin-B1 can be found in Cinnamomum verum (Ceylon cinnamon, in the rind, bark or cortex), in Uncaria guianensis (cat's claw, in the root), and in Vitis vinifera (common grape vine, in the leaf)[3] or in peach.[4]
Procyanidin B1 can be converted into procyanidin A1 by radical oxidation using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals under neutral conditions.[5]
See also
References
- ↑ Procyanidin B1 on Sigma-Aldrich website
- ↑ Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Sano Atsushi; Yamakoshi Jun; Tokutake Shoichi; Tobe Koichiro; Kubota Yoshiro; Kikuchi Mamoru, 2003
- ↑ Proanthocyanidin-B1 on liberherbarum.com
- ↑ Postharvest sensory and phenolic characterization of ‘Elegant Lady’ and ‘Carson’ peaches. Rodrigo Infante, Loreto Contador, Pía Rubio, Danilo Aros and Álvaro Peña-Neira, Chilean Journal Of Agricultural Research, 71(3), July-September 2011, pages 445-451 (article)
- ↑ Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Kazunari Kondo, Masaaki Kurihara, Kiyoshi Fukuhara, Takashi Tanaka, Takashi Suzuki, Naoki Miyata and Masatake Toyoda, Tetrahedron Letters, 22 January 2000, Volume 41, Issue 4, Pages 485–488, doi:10.1016/S0040-4039(99)02097-3
This article is issued from Wikipedia - version of the 5/27/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.