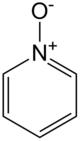
Pyridine-N-oxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pyridine-N-oxide | |||
| Other names
pyridine-1-oxide | |||
| Identifiers | |||
| 694-59-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:29136 | ||
| ChemSpider | 12229 | ||
| ECHA InfoCard | 100.010.705 | ||
| PubChem | 12753 | ||
| |||
| |||
| Properties | |||
| C5H5NO | |||
| Molar mass | 95.101 | ||
| Appearance | colourless solid | ||
| Melting point | 65 to 66 °C (149 to 151 °F; 338 to 339 K) | ||
| Boiling point | 270 °C (518 °F; 543 K) | ||
| high | |||
| Acidity (pKa) | 0.8 (of conjugate acid) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peracids as the oxidising agent.[1] The molecule is planar. The compound is used infrequently as an oxidizing reagent in organic synthesis.[2] It also serves as a ligand in coordination chemistry.
Synthesis and reactions
The oxidation of pyridine is effected with peracetic acid, a reaction that affords the protonated derivative. Subsequent treatment with base liberates the neutral oxide.[3]
Pyridine N-oxide is about 105 less basic than pyridine. It does however form an isolable hydrochloride [C5H5NOH]Cl. Chlorination of the N-oxide with phosphorus oxychloride gives 4- and 2-chloropyridines.[4]
Safety
The compound is a skin irritant.
References
- ↑ J. Meisenheimer (1926). "Über Pyridin-, Chinolin- und Isochinolin-N-oxyd". Berichte der deutschen chemischen Gesellschaft. 59 (8): 1848–1853. doi:10.1002/cber.19260590828.
- ↑ S. Nicholas Kilényi "Pyridine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis, 2001 John Wiley & Sons, New York. doi:10.1002/047084289X.rp283 Article Online Posting Date: April 15, 2001
- ↑ H. S. Mosher, L. Turner, and A. Carlsmith (1963). "Pyridine-N-oxide". Org. Synth.; Coll. Vol., 4, p. 828
- ↑ E. F. V. Scriven "Pyridines and their Benzo Derivatives: (ii) Reactivity at Ring Atoms" in Comprehensive Heterocyclic Chemistry 1984, Volume 2, Pages 165–314. doi:10.1016/B978-008096519-2.00027-8