Scorpion toxin
| Scorpion long-chain toxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
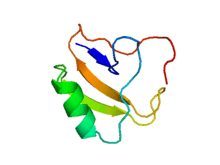 Crystal structure of toxin II from the scorpion Androctonus australis Hector.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Toxin_3 | ||||||||
| Pfam | PF00537 | ||||||||
| InterPro | IPR002061 | ||||||||
| SCOP | 2sn3 | ||||||||
| SUPERFAMILY | 2sn3 | ||||||||
| OPM superfamily | 61 | ||||||||
| OPM protein | 1djt | ||||||||
| |||||||||
| Scorpion short toxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Toxin_2 | ||||||||
| Pfam | PF00451 | ||||||||
| Pfam clan | CL0054 | ||||||||
| InterPro | IPR001947 | ||||||||
| PROSITE | PDOC00875 | ||||||||
| |||||||||
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal or insect specific, and acts by binding to sodium channels, inhibiting the inactivation of activated channels and blocking neuronal transmission.
The family includes related short- and long-chain scorpion toxins. It also contains a group of proteinase inhibitors from the plants Arabidopsis thaliana and Brassica spp.
The Brassica napus (Oil seed rape) and Sinapis alba (White mustard) inhibitors,[3][4] inhibit the catalytic activity of bovine beta-trypsin and bovine alpha-chymotrypsin, which belong to MEROPS peptidase family S1 (InterPro: IPR001254).[5]
This group of proteins is now used in the creation of insecticides, vaccines, and protein engineering scaffolds.
Structure
The complete covalent structure of several such toxins has been deduced: They comprise around 66 amino acid residues forming a three stranded anti-parallel beta sheet over which lies an alpha helix of approximately three turns. Four disulfide bridges cross-link the structure of the long-chain toxins whereas the short toxins contain only three.[6][7] BmKAEP, an anti-epilepsy peptide isolated from the venom of the Manchurian scorpion,[8] shows similarity to both scorpion neurotoxins and anti-insect toxins.
Function
The toxin's molecular function is to inhibit ion channels. Scorpion toxins are used in insecticides, vaccines, and protein engineering scaffolds. The toxins are now used to treat cancer patients by injecting fluorescent scorpion toxin into cancerous tissue to show tumor boundaries. Scorpion toxin genes are also used to kill insect pests by creating hypervirulent fungus in the insect through gene insertion.
Subfamilies
References
- ↑ PDB: 1PTX; Housset D; Habersetzer-Rochat C; Astier JP; Fontecilla-Camps JC (April 1994). "Crystal structure of toxin II from the scorpion Androctonus australis Hector refined at 1.3 A resolution". J. Mol. Biol. 238 (1): 88–103. doi:10.1006/jmbi.1994.1270. PMID 8145259.
- ↑ Krezel, A. M.; Kasibhatla, C.; Hidalgo, P.; MacKinnon, R.; Wagner, G. (1995). "Solution structure of the potassium channel inhibitor agitoxin 2: Caliper for probing channel geometry". Protein Science. 4 (8): 1478–1489. doi:10.1002/pro.5560040805. PMC 2143198
 . PMID 8520473.
. PMID 8520473. - ↑ Ronchi S; Ceciliani F; Ascenzi P; Bortolotti F; Menegatti E; Palmieri S (1994). "Purification, inhibitory properties, amino acid sequence and identification of the reactive site of a new serine proteinase inhibitor from oil-rape (Brassica napus) seed". FEBS Lett. 342 (2): 221–224. doi:10.1016/0014-5793(94)80505-9. PMID 8143882.
- ↑ Bolognesi M; Ronchi S; Tedeschi G; Ascenzi P; Bortolotti F; Menegatti E; Palmieri S; Thomas RM (1992). "Purification, inhibitory properties and amino acid sequence of a new serine proteinase inhibitor from white mustard (Sinapis alba L.) seed". FEBS Lett. 301 (1): 10–14. doi:10.1016/0014-5793(92)80199-Q. PMID 1451776.
- ↑ Rawlings ND; Barrett AJ; Tolle DP (2004). "Evolutionary families of peptidase inhibitors". Biochem. J. 378 (Pt 3): 705–16. doi:10.1042/BJ20031825. PMC 1224039
 . PMID 14705960.
. PMID 14705960. - ↑ Granier C; Kopeyan C; Rochat H; Mansuelle P; Sampieri F; Brando T; Bahraoui EM (1990). "Primary structure of scorpion anti-insect toxins isolated from the venom of Leiurus quinquestriatus quinquestriatus". FEBS Lett. 261 (2): 423–426. doi:10.1016/0014-5793(90)80607-K. PMID 2311768.
- ↑ Rochat H; Gregoire J (1983). "Covalent structure of toxins I and II from the scorpion Buthus occitanus tunetanus". Toxicon. 21 (1): 153–162. doi:10.1016/0041-0101(83)90058-2. PMID 6845379.
- ↑ Zhou XH; Yang D; Zhang JH; Liu CM; Lei KJ (1989). "Purification and N-terminal partial sequence of anti-epilepsy peptide from venom of the scorpion Buthus martensii Karsch". Biochem. J. 257 (2): 509–517. doi:10.1042/bj2570509. PMC 1135608
 . PMID 2930463.
. PMID 2930463.
External links
This article incorporates text from the public domain Pfam and InterPro IPR002061